INTRODUCTION
Many definitions have been proposed for probiotic cultures depending upon their mechanism of action. Probiotics can be defined as “a preparation of, or a product containing viable, defined microorganisms in sufficient numbers, which alter the microflora within a compartment of the host and thus exerting beneficial health effects in the host”.1 The definition proposed in the 2001 report of a Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) Expert Consultation on “Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria” is probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.2
According to Von Wright3 probiotics are “a mono- or mixed culture of live micro-organisms which, when applied to man or animals, beneficially affect the host by improving the properties of indigenous microflora”. Although this concept was introduced in the early 20th century, studies relating to probiotics have continued until now to accurately determine the applicability of some well known strains, such as Lactobacillus and Bifidobacterium to human health that affect glucose homeostasis and reduce inflammation,4 bioavailability of the cultures and secondary effects that they could cause inside the human body.
Innumerable products are available in the market that contain probiotic components and promote the presence of probiotic cultures in the food. Dairy products probably contain the greatest number of probiotic materials, and at the greatest level, that provide a peculiar flavor and an increase of shelf life.5
Many benefits have been promoted in relation to the use of probiotic cultures but there is not much information in literature relating to whether probiotics could cause any kind of damage in a short or long term in our bodies resulting from their consumption. For that reason, clinical testing of probiotics using protocols similar to the study of pharmaceutical agents has established a reasonable doubt relating to the veracity of the beneficial effects of some strains, but other studies have proven probiotic efficacy based upon more rational approaches that will lead to the right selection of the strains to foods to make them functional and with an added-value.3
The fact that most people consume probiotic products without discrimination constitutes a special concern because in most cases people use these food products for medicinal purposes. People who suffer from a wide variety of ailments find in probiotics to be a simple way to complement their clinical therapy without understanding how these materials may work. Furthermore, others consume them for preventive reasons.6 The interest in this area is growing as well as studies of the safety and quality criteria of probiotics for human use. These studies treat probiotics more or less like a drug, and the recommendations that are provided for the use of the probiotic are closely linked to regulations which must establish dose-response effect as a requirement to assure through them their effectiveness and safety action as a functional food.3
PROBIOTICS OF INTEREST AND HEALTH BENEFITS
Many probiotics exist and as more are studied, more benefits have been found, but these positive effects are strain specific and the positive effect of one strain cannot be necessarily be attributed to others.6
Certain probiotics have alleged preventive and sometimes curative effects against certain diseases,1 and these will be discussed next (Figure 1).
Some probiotics are listed here in Table 1.
| Table 1: Commonly used probiotics.1 |
| Probiotics |
| Lactobacilli
Lactobacillus acidophilus
L. delbrueckii (subsp bulgaricus)
L. brevis
L. cellobiosus
L. curvatus
L. fermentum
L. plantarum
L. casei
L. rhemnosus
L. reuteri
L. gasseri
Yeast
Sacchromyces boulardii
S. cerevisiae
|
Gram Positive Cocci
Lactococcus lactis (subsp cremoris)
Enterococcus faecium
Streptococcus salivarius (subsp thermophilus)
Streptococcus diactylactis
Streptococcus intermedius
Bifidofacteria
Bifidobacterium bifidum
B. adolescentis
B. animalis
B. infantis
B. longum
B. thermophilum
B. breve |
Some beneficial health benefits of probiotics are summarized in Figure 1.
Figure 1: Health benefits of probiotics.7
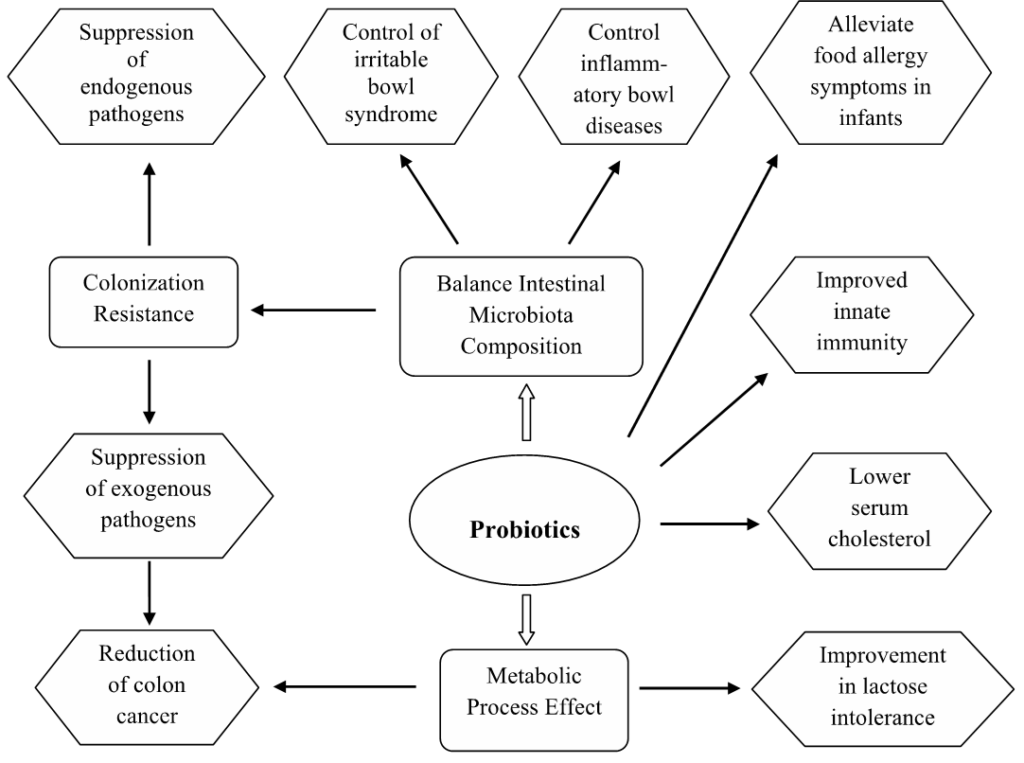
Diarrhea
Some studies have reported the action of the probiotic cultures in prevention and treatment of several kinds of diarrhea. According to Kumar, et al.1 diarrhea is a common adverse effect from a microbial imbalance resulting from antibiotic therapy. Von Wright and Salminen8 reported that different preparations containing lactobacilli, enterococci and bifidobacteria have shown to prevent, alleviate or cure different types of diarrheas. Guarino, et al9 concluded that there is a solid evidence that probiotics have some efficacy in prevention of American Academy of Dermatology (AAD), but the number needed to treat is an issue.
Constipation
Probiotics seem to have beneficial effects on symptoms of constipation, and are associated with low risk of adverse events; although the evidences are inconsistent. Their benefits may occur directly or indirectly from their effect on the composition and/or activity of the gut microbiota, and/or stimulation of the immune system.10
Lactose Intolerance
Kumar, et al1 cited that lactose intolerance can result because of “either a congenital deficiency of the intestinal mucosal enzyme β-galactosidase or a reduction in lactose activity due to intestinal disorders like gastroenteritis”. To that respect, there is some evidence about the important role of live probiotic bacteria such as Acidophilus whose presence in yogurt, improved the lactose digestion and tolerance. Regarding the benefit effect on lactose malabsorption, Von Wright and Salminen8 added that this also could be caused due to the longer transit time of the semisolid fermented milk products in comparison with normal milk.
Urinary Tract Infections (UTI)
The effect of probiotics was evaluated in a topical form, and whether probiotics can be effectively used in an oral form is pending further study.1
Cancer
Some probiotics have the ability to reduce the incidence of dimethyl hydrazine-induced colonic tumors based upon results from animal studies. Also, positive effects of L. casei have been reported in relation to the superficial bladder cancer. Furthermore, a specific type of L. acidophilus was found to lower amounts of extractable fecal and urinary mutagens in humans.8
Other Benefits
These include their action against allergic conditions, inflammatory bowel disease, and irritable bowel syndrome. Moreover, some studies found that probiotics act in favor of the immune system, help to reduce the serum cholesterol in humans, and few trials evaluated their efficacy related to vaginal infections.1,8,11
In addition, Rautava, et al12 investigated and concluded that microbial contact in utero is associated with changes in fetal intestinal innate immune gene expression profile and the innate immune gene expression profiles in the placenta and the fetal gut may be modulated by dietary supplementation with specific probiotics. Peluchi, et al13 undertook a meta- analysis of randomized controlled trials to investigate whether probiotic use during pregnancy and early life decreases the incidence of atopic dermatitis and Immunoglobulin E (IgE) associated atopic dermatitis in infants and young children. Investigators concluded that probiotics have a moderate role in the prevention of atopic dermatitis and IgE-associated atopic dermatitis in infants.
PROBIOTIC STUDIES – VALUE OF EFFICACY EVIDENCE
Salminen and Gueimonde14 referred to the invaluable importance of scientific studies to validate health potential of probiotics. “Knowledge of the mechanisms is an important factor, complemented with target functions and biomarkers that are accepted as relevant to the state of health and well-being and/or reduction in the risk of disease”.
Many of the initial in vitro studies have been conducted using cell culture models or in vivo using animal models prior to the initiation of clinical trials in human subjects. According to Sanders, et al.,15 studies in vitro are considered too simplistic and many times do not mimic exactly the conditions in humans, thus it is difficult to predict the safety of the compounds analyzed. Considering that, it is recommended not to extent the results made in vitro studies until these can be corroborated in vivo. The results obtained under in vitro tests would provide the first step of screening for probiotic safety and efficacy and could include: genomic analysis, Deoxyribonucleic acid (DNA) based and phenotypic strain identification and measurement of viability.
In relation to in vivo studies, there are animal and human systems that have been developed. “Numerous animal model systems have been developed and are used widely for the study of physiological effects of a wide diversity of bioactive components and diets”. Those results are not exactly reproducible in humans, but they are a preliminary substantiation of safety for further studies in humans. These last ones mostly reflect peculiar effects in a specific condition and are not representative of the general population. As in vitro and in animal studies, the results obtained with humans have to be manipulated with caution to avoid overextending the meaning of the results to the entire population. These studies should be confirmed by well-designed, randomized, double blind, controlled trials, which become complicated since it is necessary to define the active ingredients of the product as it is sold in the market, that can be difficult considering that product could experiment changes during its shelf life.15
SURVIVAL AND GROWTH OF PROBIOTICS IN THE GASTROINTESTINAL TRACT (GIT)
The survival of probiotics, when they are in transit, is important considering that they can affect the composition and behavior of the intestinal microflora.16 Also, their survival is crucial if there is to be a benefit effect of the probiotics cultures inside our body. The probiotic must survive for a considerable amount of time to let them interact with the gut microflora and the host. i.e. L. rhamnosus was evaluated and its counts reduce gradually after the end of administration. It disappears below the detection levels in the fecal samples within approximately 2 weeks that probably means the ability of the strain to survive and multiply in the gut.8
In vitro studies have been developed to mimic the stress caused by the low gastric pH, bile acid and pancreatic juice; and evaluate how the probiotic cultures react to this environment. The viability of doing in vivo studies to identify probiotic strains has been blocked by the difficulties of isolating them from fecal and clinical samples. Furthermore, in vivo studies have shown that “an adherent probiotic can persist on colonic mucosa several days after it has disappeared from the fecal samples”.8
Several factors can determine the degree of the survival of probiotics through the upper gastrointestinal i.e. degree of stomach pH, the length of exposure to acid, the concentration of and length of exposure to bile salts, the level of bile salthydrolase activity, species and strains used and others.16 Thus, further studies are necessary to determine the specific environment that will allow the species and probiotic strains used to withstand the rigors of the passage through the upper gastrointestinal tract and enter the colon in a viable state in sufficient amounts to cause a beneficial effect.
SAFETY OF THE EXISTING PROBIOTICS
The safety of the action must be anticipated to any risk. Only safety data is available for some traditional starter micro-organisms and for others known commercially for decades such as lactobacilli and/or bifidobacteria.3
The safety of those probiotics has been confirmed through a long period of experience with their use. Ecologically, bifidobacteria are present as the predominant bacteria in the intestinal tract of breast-fed infants and are considered to contribute to the health of infants. Until now, the safety of these microbes has not been questioned, and reports of a harmful effect of these microbes to the host are not common.17
Regarding the novel probiotics and considering the lack of benefits related to their characteristics, their safety has been deduced mainly from the common occurrence of the species either in foods or as normal commensals in the human gut.3 As Domig and others6 referred, there are no formal regulations for food-associated microorganisms and because of that, probiotic producers have made some studies, such as acute oral toxicity and test on the degradation of intestinal mucus, to ensure the “harmless” of those strains. Lactic starters have been used for a long period of time and their use never was questioned for food fermentation, but considering that there is a huge increase in the incorporation of lactic acid bacteria into foods and drugs, safety guidelines are needed to verify that those microbes are safe under their new conditions of use.
A better understanding of the positive impact as well as potential risks of probiotics must be assessed using standardized assays and thus determine drug insensitivity or resistance profiles in lactobacilli and bifidobacteria mainly, because it is a need for more statistically significant efficacy data in humans. Good manufacturing practices must be applied with quality assurance, shelf life conditions established, and labelling made clear to include minimum dosage and verifiable health claims and in addition, a regulatory framework must be established to better address probiotic issues.18,19
As cited by Doron and Snydman,20 an exhaustive report released by the Agency for Healthcare Research and Quality (AHRQ) in 2011 concluded that the current literature is not able to answer confidently questions related to the safety of probiotics in intervention studies.
REGULATORY FRAMEWORK
Government regulations differ among countries, and the status of probiotics as a component in food is currently not established on an international basis.18
In the European Union (EU), probiotics are regulated via the Novel Food Regulation (258/97/EC) that is only applied to strains that were not used before 1997, thus concerns novel foods or food ingredients. To date, only in Denmark is requiring that the relevant authority be notified by the manufacturer prior to the use of new probiotic strains. In France, a pre-market approval system for novel strains is being considered, and proposed recommendations were published by Agence Française de Se´curite´ Sanitaire des Aliments.3,21
Regulatory framework relating to food microorganisms is apparently just emerging at the EU level. In USA, a dietary supplement is defined by the Dietary Supplement Health and Education Act (DSHEA) of 1994 as a product taken by mouth that contains a “dietary ingredient” intended to supplement the diet. If a probiotic is intended for use as a dietary supplement, it is placed under the umbrella of “foods,” and as such is regulated by Food and Drug Administration’s (FDA’s) Center for Food Safety and Applied Nutrition. Under the Food, Drug, and Cosmetic Act, health claims on foods or dietary supplements can be authorized by the FDA on the basis of safety and efficacy data, or it can be Generally Recognized As Safe (GRAS). The GRAS-status is given to a probiotic when it has a history of safe use in food dating before January 1, 1958, or it has been recognized by qualified experts as safe under the conditions of intended use.3,21,22,23
However, it also means that responsibility for safety of the product resides strictly with the producer. One of the features that make this GRAS status important is that usually is restricted to a specific application and not to a general use of the organism in another context or product. For example, Bifidobacterium lactis Bb12 and Streptococcus thermophilus Th4, are considered as GRAS in the specific use in infant formula.3
According to FAO/WHO24 as also cited by Domig, et al.,6 the following guidelines for the evaluation of probiotics in food (Figure 2), are the only approach for official regulation of human food with probiotic properties.
Figure 2: Assessment of probiotics24
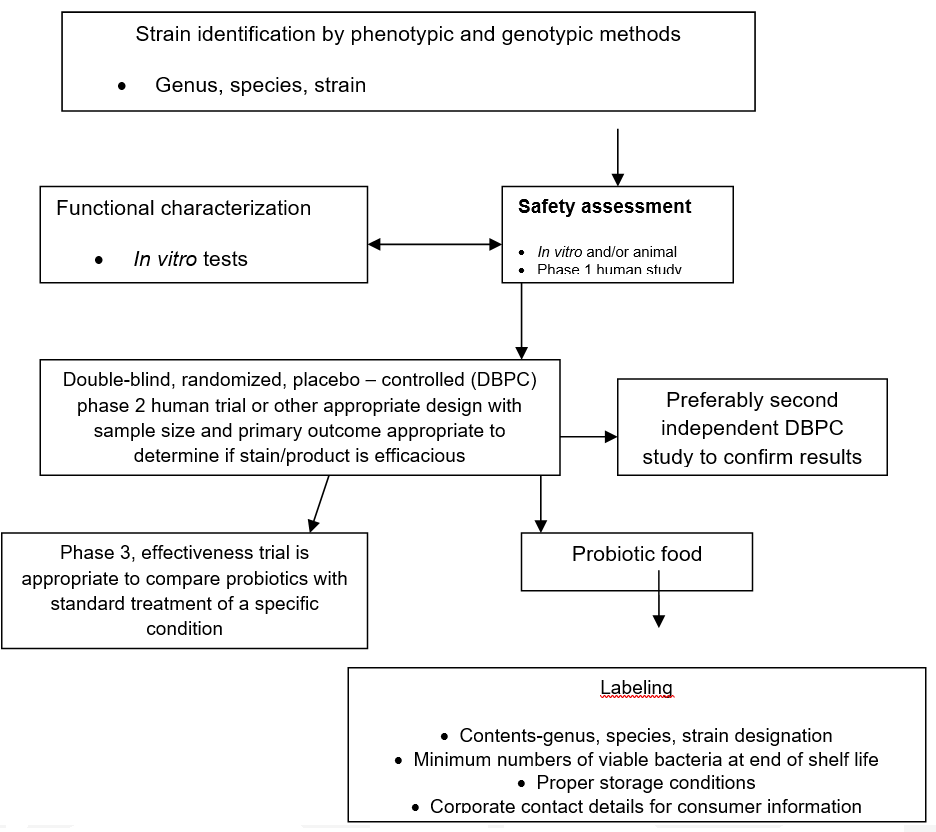
These guidelines establish the parameters required for a strain to be considered a “probiotic”. This responsibility is significant for those producers who make a profit from the sales of probiotics, and in this way, following those guidelines, will have enough support to show evidence about the health benefits that probiotics presume to have. Then, these companies by raising the required standards will be able to access the huge physician-based market and thus, will increase their profits.25
The Codex Alimentarius Commission, created in 1963 by FAO and WHO to develop food standards and guidelines has the mission to protect health of the consumers, ensuring fair trade practices, and promoting co-ordination of all food standards work undertaken by international governmental and nongovernmental organizations. Probiotic results and recommendations have been presented to 2 Codex Committees, Codex Committee on Food Labelling and Codex Committee on Nutrition and Foods for Special Dietary Uses. The FAO/WHO Consultation and the Working Group Probiotic Guidelines recommended that specific health claims be allowed where sufficient scientific evidence is available and that the product manufacturer take responsibility for ensuring that an independent third party reviews and evaluates the scientific evidence. Thus, it is expected that probiotic works may be used as a model for the Codex graft guidelines that will provide specific references to scientifically evaluate probiotics that adequately support a health claim at the same time.18,23
FUTURE RESEARCH
Additional clinical research, new well planned, long-term human clinical studies, are needed prior to the establishment of final conclusions regarding other proposed beneficial effects. Moreover, consider that each strain and product has to be documented and tested independently, extrapolation of data from closely related strains is not acceptable, and the target population must be carefully selected. This will conduct to the development of protocols of study related to functional foods.
It would also be important to determine if dairy products are the best natural vehicle for those and if those may actually help turn on probiotic activity. As an important requirement in order to guarantee safety in humans, there is a need for guidelines which describe the minimum regulations for probiotics, and additional studies that focus on the interaction of probiotics with food enzymes, vitamins, antibiotics among other elements that will determine their mechanism of action in our body. Finally, basic and applied research is urgently needed to assess the health claims made of probiotics, which should further clarify their beneficial effects.







