INTRODUCTION
The deoxyribonucleic acid (DNA) microarray technology has become a very powerful tool to explore global gene expression profiles and to measure genome-wide differences in genetic contents. Thus, the technology needs the availability of complete genome sequences of many microorganisms. Since DNA microarray measures the presence or absence of DNA regions as well as the abundance of transcripts, interpretation of the array data can be difficult in the absence of other supporting evidence. This is especially true when the physiological events are not well studied. In addition, it is not easy to sort out secondary effects caused by mutations, expression of certain genes, and different growth conditions. Consequently, the chief impact of this technology was not realized until it is combined with other high-throughput genomic methods, biochemistry, genetics, and physiology.1,2
Microarrays consist of thousands of probes (pieces of DNA ranging from 20-5000 base pairs (bp)) arrayed within a small surface area (sequences of nucleotides) that are physically and systematically bound to flat solid surfaces, slides or chips of glass, silicon or plastics,3 in known locations.4 A DNA microarray experiment consists of array fabrication, probe preparation, hybridization and data analysis.5 Although the basic array technology is the same, there are fundamental differences in its application to prokaryotes and eukaryotes. For example, total ribonucleic acid (RNA) is usually labeled for a bacterial array experiment, while poly(A) RNA is often used for eukaryotic arrays.1,6
Several applications of DNA microarray has mentioned in different areas and disciplines including disease diagnostics and characterization, evolutionary biology, pathway analysis, toxico and pharmacogenomics, forensics, and oncology. The major application of this technique was found in the field of oncology as classification of tumors, prediction of prognosis of tumors as an expression profile, single nucleotide polymorphism to study the mutation of a gene. Also, to study the gene amplification, deletion, identify the copy number of genes using comparative genomic polymorphism. Re-sequencing arrays are used to identify the somatic mutation in cancers by sequencing a portion of the genome.7
Microarrays are powerful tools among the latest technologies for rapid, precise, reliable, and efficient for detection and diagnosis of a high quantity of pathogens causing diseases in animals and humans.8 In the veterinary field, only a few studies were done to interrogate the gene expression profile of pathogens.7 Early diagnosis of an infectious disease is always desirable to prevent its spread among livestock species and thus reduce the economic losses both to the livestock owners and the country as a whole. Microarray was used to study host-pathogen interactions and the diagnosis of infectious pathogens. The genes responsible for several stages of cell to cell interaction of pathogens from adhesion to entry into the host cell and evading the host immune mechanism can be studied.9
Accordingly, DNA microarrays open a new way for the parallel detection and analysis of the patterns of expression of thousands of genes (currently about 20,000-40,000) in a single experiment. The high capacity for data generation with microarraybased approaches have time and resource advantages. It allows the study of the interaction between different molecular pathways. This is a major advantage since any disease process is likely to involve the coordinated interaction of several functional pathways involving multiple proteins, thus reflecting the expression of many genes. In infectious diseases, microarray-based approaches allow researchers to study the entire host and pathogen genomes. Thus, these approaches provide insight into the functional responses of both host and pathogens. Although the technology is expensive, it already has the lowest cost per assay of modern nucleic acid technologies, and will inevitably become cheaper in the future.7,10,11
HISTORICAL PERSPECTIVES OF DNA MICROARRAY
In the late 1970s, the introduction of the first hybridization technique was reported as dot blots. In addition, the microarray technology has the advantage of the idea to use multiple DNA libraries arrayed on filters to hybridize with cloned sequences and proved to be the base for the development of high-density microarrays.3,4
In the mid of 1990s, the microarray technique evolved from the southern blotting technique and it is first commercialized in 1994 by Ron Davis and Patrick Brown of Stanford University and later it was also commercialized in 1996 and in 1997 for expression of the whole genome of the eukaryotic cell (Saccharomyces cervisiae). In 1999, Todd Golub and colleagues used microarrays for the first time to classify cancers. In 2002, when there is a havoc of severe acute respiratory syndrome (SARS) was identified as an unknown coronavirus by using microarray. The chip used had conserved probes for virus genera; hence the virus was identified as an unknown coronavirus. The chip used for the SARS-corona virus was subsequently developed as a full-fledged microarray chip for virus diagnosis and was given the name Virochip.7,12,13
In 2004, a Roche Company released Amplichip CYP450, the first Federal Drug Authority (FDA)-approved microarray for diagnostic purposes. In 2012, a chip was designed for the first time by Indian Veterinary Research Institute (IVRI) scientists in the division of biochemistry for the detection of viral diseases affecting livestock’s.14,15
OVERVIEW ON PRINCIPLE OF DNA MICROARRAY TECHNOLOGY
DNA Microarray
A microarray is a collection of microscopic spots arranged in an array on a grid-like format and attached to a solid surface or membrane. Each individual spot is present at a precisely defined location on the substrate. In DNA microarray, these spots are single-stranded DNA fragments known as probes. These probes hybridize with a specific nucleic acid sequence called target which is labeled with a fluorescent dye. The extent of binding between the target and probe is quantified by measuring the signal emitted by labeling dye when scanned. Accordingly, utilizing the same basic principle of southern blotting, microarray analysis permits the simultaneous detection and expression studies of several genes on a single chip.4,16
DNA microarrays simply consist of small and solid supports onto which the sequences from thousands of different genes are attached at fixed locations. Hence, they are usually glass microscope slides, the size of two side-by-side pinky fingers, but can also be silicon chips or nylon membranes. The DNA is printed, spotted, and actually synthesized directly onto the support.4,17,18
The immobilization of DNA probes can be defined as the attachment of DNA molecules to a surface resulting in reduction or loss of mobility that is essential to develop a whole range of microarrays. The way in which DNA is immobilized determines the property of a microarray. The physicochemical properties of both surface and DNA probes are determinant factors in the selection of a suitable immobilization strategy. Even though microarray can be developed by different strategies, the immobilization of the DNA probes on the surface has a common critical step that is the probes can be made base-by-base to the support or presynthesized and then spotted on the surface. Many immobilization techniques have been developed in the past years, which are mainly based on three important mechanisms: (A) physical adsorption; (B) covalent immobilization; and (C) streptavidin-biotin immobilization. In order to achieve high sensitivity and selectivity of the probes, the probes should have minimum non-specific adsorption and stable immobilized DNA. The control of this step is essential to ensure high reactivity, orientation, accessibility, and stability of the surface-confined probe and to avoid non-specific binding.19,20
Microarrays can be fabricated by several methods, including printing with fine-pointed pins onto glass slides (spotting), photolithography using pre-made masks, photolithography using dynamic micromirror devices, ink-jet printing, and soft lithography also known as molecular stamp (in situ synthesis). Microarrays fabricated by in situ synthesis provide higher density, better reproducibility and little batch to batch variation than those fabricated by spotting technique, but much higher cost. In spotted microarrays, the probes are oligonucleotides, complementary DNA (cDNA) or Polymerase chain reaction (PCR) products. The probes are synthesized and then “spotted” onto the glass slide. In the case of experimental or clinical samples, the nucleic acid within the probes matrix has prepared probes and is ready to capture complementary RNA (cRNA) targets. This technique is used to produce “inhouse” microarrays according to the different experimental designs easily. This provides a relatively low-cost microarray that may be customized for each study and avoids the costs of purchasing expensive commercial microarrays.4,21
Once the microarrays are constructed, hybridization and detection are relatively simple and rapid, allowing real-time data analysis in field-scale heterogeneous environments.22,23 Microarray technology is amenable to automation and therefore, has the potential of being cost-effective compared to traditional hybridization methods.4,8,24
Principle of DNA Microarray
The core principle of DNA microarray is hybridization property between the nucleotides where complementary nucleotides specifically pair with each other by forming the hydrogen bond between base pairs. A high number of complementary base pairs in a nucleotide sequence mean tighter the non-covalent bonding of base pairs. After washing off, the tightly paired strand remains hybridized. Fluorescently labeled target sequences bind to probe and generate signals. The signal strength depends on the amount of target sequence bound to the probe. The relative intensity of a spot is compared to the intensity of another in different conditions.7,14
During hybridization DNA ‘targets’ diffuse passively across the glass surface, then sequences complementary to a probe will anneal and form a DNA duplex. Hybridized targets can then be detected using one of many reporter molecule systems. In essence, a microarray is a reverse dot blot that employs the same principle of hybridization and detection used for many years with membrane-bound nucleic acids (e.g. Southern and Northern blots).5
Types of DNA Microarrays
Microarrays can be broadly classified according to at least three criteria: 1) length of the probes; 2) manufacturing method; and 3) the number of samples that can be simultaneously profiled on one array. According to the length of the probes, arrays can be classified into “cDNA arrays,” which use long probes of hundreds or thousands of base pairs (bps), and “oligonucleotide arrays,” which use short probes (50 or less bps). Manufacturing methods include “deposition” of previously synthesized sequences and “in situ synthesis”.22,25
Usually, cDNA arrays are manufactured using deposition, while oligonucleotide arrays are manufactured using in situ technologies. In situ technologies include “photolithography” (Affymetrix®, Santa Clara, California, USA), “inkjet printing” (Agilent®, Palo Alto, California, USA), and “electrochemical synthesis” (Combimatrix, Mukilteo, Washington, USA). With regard to the number of samples that can be profiled on one array, a single sample can be analyzed using single-channel arrays whereas two or more samples can be analyzed using multiple-channel arrays simultaneously. Affymetrix GeneChip® is a sample for an oligonucleotide and single-channel array.26,27
Spotted microarrays: The first widely and broadly available array platform were spotted microarrays and originated in the laboratory of Patrick Brown. Spotted also called cDNA microarrays, take their name since probes are synthesized apart and printed mechanically on the slide. The PCR products or long oligonucleotides on the glass microscope slides are printed using a robot equipped with nibs capable of wicking up DNA from microtiter plates and depositing it onto the glass surface with micron precision.17,28 The advantages of glass cDNA microarrays include their relative affordability, no specific and specialized equipment for hybridization, increased detection sensitivity due to longer target sequences (2 kbp) and primary sequence information is not needed to print a DNA sequence. However, this technique is not specific as an oligonucleotide microarray and It requires intensive labor requirement for synthesizing, purifying, and storing DNA solutions before microarray fabrication. Besides, it fails to specifically detect individual homologies genes sequence between clones representing different closely related members of the same gene family.16
Spotted microarrays are primarily a comparative technology and used to examine the relative concentrations between two target samples. Complex samples to be compared are labeled with uniquely colored fluorescent tags before being mixed together and allowed to compete for hybridization to the microarray spots. In this way, differences between the samples are observed on a per spot basis because the fractional occupancy of the spot hybridized by each sample reflects the relative concentration of the gene or target in the original complex sample. As a result, spotted microarrays are often called two-color or two-sample arrays.4,7,12
Oligonucleotide arrays: Oligonucleotide microarrays are highly specific, hybridize with only a single sample and thus give absolute expression level of the sample concerned. In this method, arrays are constructed by synthesizing single-stranded oligonucleotides in situ by using a photolithographic technique to generate high density (>280, 000 features) microarray chips. In this system collection and storage of cloned DNA and PCR product is not required.2,16 Oligonucleotide array is limited to gene expression and analysis and requires a large amount of biological material.14
The advantages offered by the in situ oligonucleotide array format include speed, specificity, and reproducibility. Some of the disadvantages of in situ oligonucleotide array format are listed below. Firstly, in situ oligonucleotide array formats tend to have expensive specialized equipment e.g. to carry out the hybridization, staining of label, washing, and quantization process. Secondly, the ready-made product (GeneChips®) is expensive. Thirdly, in contrast to glass cDNA microarrays, short-sequences have decreased sensitivity/binding and reduced flexibility. However, the low sensitivity can be overcome by using multiple probes. Besides, in some circumstances, the types of equipment and facilities for DNA array productions, hybridization, and detection are restricted to centralized manufacturers, thus limits the researcher’s flexibility.29
PREPARATION OF DNA MICROARRAY
The microarray preparation is a multi-stage process that requires accuracy and understanding in each individual step that may influence the gene expression estimates. In order to increase the accuracy of the experiment, the condition of the biological materials is controlled at various steps. The procedure used in a microarray experiment is very similar across different platforms.15
Microarray technology works on three important steps, with which a comprehensive understanding of the cell/organism under study can be achieved through microarray preparation, probes preparation followed by hybridization and finally, scanning, imaging and data analysis.4,30 The power of a DNA microarray lies in the fact that is there may be many thousands of different DNA molecules bonded to an array that makes it possible to measure the expression of thousands of genes in a sample simultaneously at one time.12
Preparation of DNA microarrays involves several-step including obtaining of the DNA sequences, designation of oligonucleotides or primers for generating probe DNA, selection and preparation of suitable glass surface and depositing the probe DNA on its surface.6 DNA microarrays are made either by chemically synthesizing DNA probes on a solid surface or by attaching a pre-made DNA probe to a solid surface.31 The three primary technologies currently used in automated microarray production are photolithography, mechanical micro spotting and inkjets.14,26,32
Microspotting Technique
The fabrication microarray was invented by Patrick Brown of Stanford University, in which the microspotting technique relies on direct surface contact. In this method, long DNA molecules (cDNA) are deposited by high-speed robots on a solid surface. Solid and hollow (split-open) pen designs are used to transfer target nucleic acid onto the supporting surface. The pen is dipped into the target solution and a small volume of the solution adheres to the pen. When the pen comes into contact with the supporting surface, it transfers a fraction of nucleic acid solution onto solid surfaces (Figure 1).32
Figure 1. Microarray Microspotting Technique
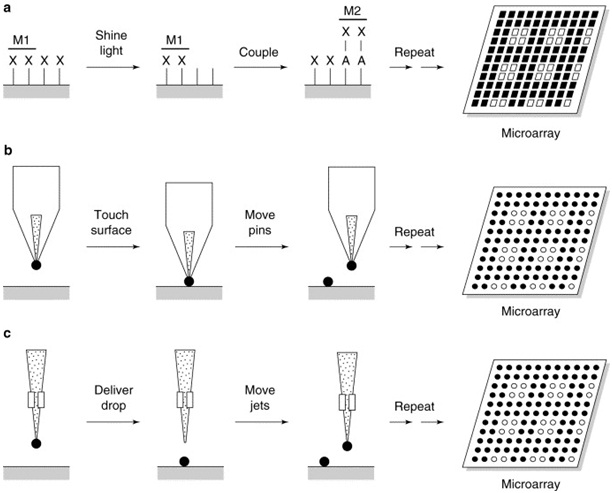
The most commonly used microarray fabrication technology is mechanical microspotting, which uses direct contact of computer-controlled multiple pins, tweezers, or capillaries to deliver picoliter volumes of pre-made biochemical reagents (e.g., oligonucleotides, cDNA, genomic DNA, antibodies, or small molecules) to a solid surface. Currently, more than 1,000 individual cDNA molecules can be deposited in an area of 1 cm2 using this technology.33 The advantages of microspotting include ease of implementation, low cost, and versatility, while a major disadvantage is that each sample to be arrayed must be prepared, purified, and stored prior to microarray fabrication. In addition, microspotting rarely produces the densities that can be achieved with photolithography.25
Photolithography Technique
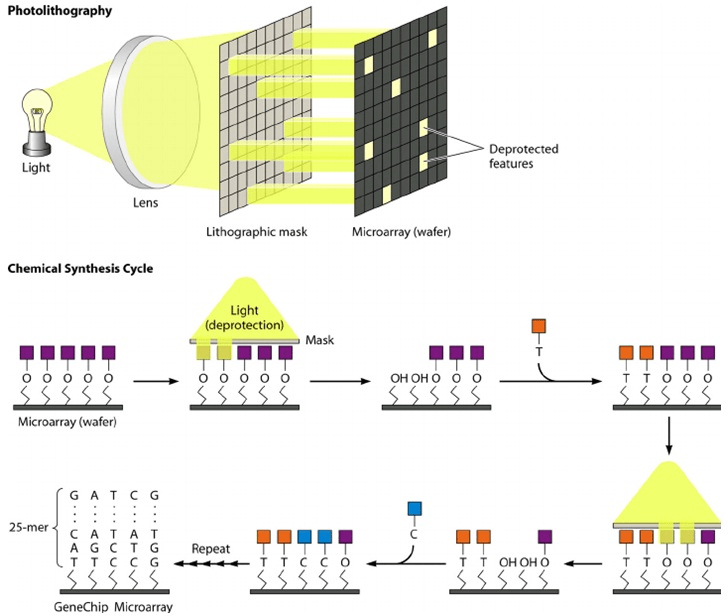
The photolithography approach uses the same technology for making semiconductor chips. DNA microarrays are fabricated mostly onto glass or plastic wafers or are placed in tiny glass tubes and reservoirs. In photolithography, oligonucleotides are synthesized in situ on a solid surface in a predefined spatial pattern by using a combination of chemistry and photolithographic methods borrowed from the semiconductor industry. Briefly, a glass or fused silica substrate is covalently modified with a silane reagent to obtain a surface containing reactive amine groups, which are then modified with a specific photo protecting group, namely methylnitro-piperonyl-oxy-carbonyl (MeNPOC).4,35
The surface of the specific regions are then activated through exposure to light, and a single base is added to the hydroxyl groups of these exposed surface regions using a standard phosphoramidite DNA synthesis method. The process of photoprotection and nucleotide addition is repeated until the desired sequences are generated. Typically, the probes synthesized in situ on the arrays are 20-25 bp in length. Since the average stepwise efficiency of oligonucleotide synthesis ranges from 90-95%, the proportion of the full-length sequences for 20-mer probes is approximately 10%. However, this should have a relatively minor effect on the performance of microarray hybridization because of the high absolute amount of full-length probes on the support.36
A supporting surface is covered with a photoactive mask, and when lights are selectively rayed through masks reactant groups are exposed and can react with the following units. In each step (Figure 2), the unprotected areas are first activated with light which removes the light-sensitive protective groups. Exposure of the activated area results in chemical attachment of the nucleoside base to the activated positions. A new mask pattern is applied. This process is then repeated and a new nucleotide has been added to the oligomers.32
Figure 2. Microarray Manufacturing Using Photolithography
Ink-jetting Technology
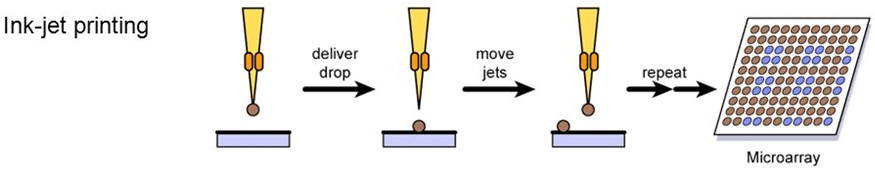
Ink-jet ejection technologies provide another means of fabricating microarrays. In this approach, the sample is taken from the source plate, and a droplet of the sample is ejected from the print head onto the surface of the substrate. Similar to microspotting, ink-jet ejection allows the spotting of virtually any biological molecule of interest, including cDNA, genomic DNA, antibodies, and small molecules. In contrast to microspotting, ink-jets have the advantage of avoiding direct surface contact but cannot be used to manufacture microarrays as dense as those prepared by photolithography or microspotting approaches. Currently, two types of non-contact ink-jet print technologies, piezoelectric pumps and syringe-solenoid, are used for printing microarrays.14,25,32
Ink-jetting is a biochemical sample is loaded into a miniature nozzle equipped with a piezoelectric fitting (rectangles) and an electrical current is used to expel a precise amount of liquid from the jet onto the substrate. After the first jetting step, the jet is washed and a second sample is loaded and deposited to an adjacent address. A repeated series of cycles with multiple jets enable rapid microarray production. Ink-jetting Technology utilizes piezoelectric and other forms of propulsion to transfer biochemical substances from miniature nozzles to solid surfaces (Figure 3). Similar to the microspotting approaches, drop-on-demand technologies allow high-density gridding of virtually any biomolecule of interest, including cDNAs, genomic DNAs, antibodies and small molecules. Ink-jetting technology is being developed at several centers including Incyte Pharmaceuticals (Palo Alto, California, USA) and Protogene (Palo Alto, California, USA).19,37
Figure 3. Microarray Manufacturing Using Ink-Jet Printing
APPLICATION OF DNA MICROARRAY IN DETECTION OF PATHOGENS AND ANIMAL DISEASE DIAGNOSIS
Pathogens adapt rapidly to a changing environment whether outside or within the host through the regulation of expression of several genes, including virulence genes, to the changing stimuli. The microarrays allow global analysis of such gene expression simultaneously under a particular condition.1,9
In veterinary diagnostics, the microarray technology is in the stage of dynamic development with many alternatives and choices available. It has the potential to identify and detect agents of interest at the serotype or subspecies level, or to differentiate agents that cause similar or particular lesions or signs.14,39 Initially, microarray directed at mapping, gene expression studies or single nucleotide polymorphisms (SNPs)/point mutation analysis on large scale.2,4,34 Hence, it is used to detect a wide variety of veterinary pathogens and also familiar for genetic and forensic applications, pharmacogenomic studies, cancer research, and diagnostics, and for infectious and genetic disease diagnostics purposes. Besides, they are popular and used for many proteomic studies cell and tissue-based applications.3
The main goal of the microarray technique is to successfully detect pathogens before the onset of the disease, especially those pathogens associated with the outbreak of the disease. However, the development and use of species-specific primers are impractical for the routine analysis of clinical samples containing several pathogens. The 16S ribosomal DNA (rDNA) has regions that are highly conserved and variable in most bacteria; therefore could be used to facilitate the diagnosis of infectious bacterial diseases.40
According to Huang et al, Easy Operating Pathogen Microarray (EOPM) is a high quantity pathogen microarray platform for large scale pathogen surveillance, including viral sequences representing complete and partial viral genomes, 2,110,258 bacterial 16S ribosomal RNA (rRNA) sequences, 621,351 fungal 18S rRNA sequences, and 1,735,744 18S rRNA sequences from parasites. The EOPM Chip distinguishes all 2,554 known vertebrate virus species (involving 151 genera, 36 families), 124 bacterial genera (involving 53 families), 38 fungal genera (involving 17 families), and 47 genera of parasites (involving 24 families). Whereas bacterial 16S rRNA genes showed a relatively high-level of homology, and that bacteria require the presence of active virulence genes for pathogenesis. DNA microarrays can be applied to detect multiple pathogens with the use of specific DNA sequences viz. 16S rDNA sequences can be designed for characterization and detection of the taxonomy of pathogens.35,41,42
Similarly, Peterson et al43 has also developed a highly specific and reliable spotted array for bacterial pathogens, consisting of 489 70mer probes to detect 40 bacterial pathogens of medical, veterinary and zoonotic importance including 15 National Institute of Allergy and Infectious Diseases (NIAID) of Category A, B and C pathogens and also associated genes that encode resistance for antimicrobial. The array is also identified DNA elements that are important for horizontal gene transfer among bacteria by validating multi-drug resistant (MDR) pathogenic bacteria as pure cultures or by following their inoculation in complex and highly organic sample matrices, such as soil and manure. In addition, Shallom and his colleagues introduced a new species for independent forensics array-based technology for rapid and adequate identification of biological threat agents and newly emerging infectious pathogens.44
Application in Virus Detection
Animal viruses probe dataset (AVPDS) is a dataset of virus-specific and conserve oligonucleotides for identification and diagnosis of viruses infecting animals that is used for microarray-based diagnosis and identification of viruses’ probes. Currently, the dataset contains 20,619 virus-specific probes for 833 viruses and their subtypes and 3,988 conserved probes for 146 viral genera.7 The first broad range virus identification chip was used for the detection of the SARS-corona virus and in the case of animals the chip used for the identification of foot and mouth disease (FMD) virus.16,45
A long oligonucleotide microarray assay was designed to identify the different types of viruses that cause vesicular or vesicular-like lesions in livestock animals. Using this microarray, the genus level of foot-and-mouth disease virus (FMDV), vesicular stomatitis virus (VSV), Swine vesicular disease virus, vesicular exanthema of swine virus (VESV), bovine alphaherpesvirus 1 (BHV1), orf virus, pseudocowpox virus, bluetongue virus serotype 1 and bovine viral diarrhea virus 1 (BVDV-1),7 and Enteroviruses46 were detected separately.
Microarrays are a suitable means for typing and multiplexed detection of various pathogens. It may be possible to multiplex ten or more PCR primer pairs on a microarray.5 The three assays for detection and typing of poultry viruses are multiplex assay for simultaneous detection of avian influenza virus (AIV) and detection and pathotyping of newcastle disease virus (NDV), and two separate assays for differentiating all AIV H and N subtypes has been developed. Consequently, it detected and tested all typed 41 AIV strains and accurately typed all high pathogenicity NDV strains. The high capacity of multiplexing different microarray assays has been exploited for detecting and subtyping all possible hemagglutinin (HA) and neuraminidase (NA) subtypes of AIV.42,47
Application in Bacterial Detection
In the field of microbiology, Pathochip was the most important diagnostic microarray chips that analyze 23S rDNA and 16S-23S rDNA intergenic spacer region (ISR) sequences of bacteria.48 Rapid tracking of the pathogen and its various strains helps in developing strategies for prevention and serotyping provides information on vaccine selection during an outbreak.45
Easy Operating Pathogen Microarray (EOPM) was a high quantity pathogen identifying array which consists of 2,110,258 bacterial 16S rRNA sequences, 621,351 fungal 18S rRNA sequences, and 1,735,744 parasitic 18S rRNA sequences.41 Later, an arraytube (AT) platform, with chromosomal and plasmid coded targets for the detection of Coxiella burnetii was also developed. Besides, it was also found suitable for detection, differentiation and genotyping of Burkholderia mallei/pseudomallei, Brucella species., Bacillus anthracis, and Chlamydia species.18
Accordingly, this helps to create a useful screening of individual pathogens having similar symptoms and multiple pathogens in co-infection.42 DNA microarray helps in typing of new emerging species/strains, and understanding evolutionary relationships among bacterial species. Toxin array for genotyping of clostridium perfringens toxins based on oligo probes of six toxins.49 DNA microarray is currently used to identify the presence of drug-resistance genes/plasmids in the infectious agents; the data thus obtained helps in developing better therapeutics.50
Application in Parasites Detection
The gene expression during the development of parasite at different stages of the life cycle reveals the unique metabolic and physical properties of that developmental stage. During toxoplasma gondii development, the transition from tachyzoites to bradyzoites shows the genes encoding metabolic enzymes and bradyzoite secretory antigens were upregulated.51 Among different parasitic diseases affecting the livestock, differentially expressed genes of Eimeria species were studied. Hence, DNA microarray used to study the molecular basis of sporulation and invasion of the precocious line of eimeria maxima.12,42,52
LIMITATION AND NEGATIVE IMPLICATIONS OF MICROARRAYS
Despite their incredibly wide variety of applications, microarrays have a number of limitations. Firstly, arrays provide an indirect measure of relative concentration. That is the signal measured at a given position on a microarray is typically assumed to be proportional to the concentration of a presumed single species in solution that can hybridize to that location. However, due to the kinetics of hybridization, the signal level at a given location on the array is not linearly proportional to the concentration of the species hybridizing to the array. At high concentrations, the array will become saturated and at low concentrations, equilibrium favors no binding. Hence, the signal is linear only over a limited range of concentrations in solution.14,52
Secondly, especially for complex mammalian genomes, it is often difficult to design arrays in which multiple related DNA/ RNA sequences will not bind to the same probe on the array. A sequence on an array that was designed to detect “gene A”, may also detect “genes B, C, and D” if those genes have significant sequence homology to gene A. This can particularly problematic for gene families and for genes with multiple splice variants. However, it is difficult to design arrays that will uniquely detect every exon or gene in genomes with multiple related genes.19,53
Lastly, a DNA array can only detect sequences that the array was designed to detect. That is if the solution being hybridized to the array contains RNA or DNA species for which there is no complementary sequence on the array. Thus, those species that are not included in gene expression analysis will not be detected and represented on the array. Moreover, for highly variable genomes such as those from bacteria, arrays are typically designed using information from the genome of a reference strain. Such arrays may be missing a large fraction of the genes present in a given isolate of the same species.1,4,19
CONCLUSION
Microarray technology generally provides a highly efficient and rapid method of analysis for varieties of disciplines and fields. Besides, it is rapidly developing and evolving technology that incorporates biology, automation, and informatics. Protein Arrays and tissue arrays are becoming more broadly available and accepted techniques representing highly interdisciplinary and synergist efforts. New applications are being pursued beyond gene expression, gene discovery, and pharmacogenomics. Accordingly, microarray technology is now gaining prime importance in the areas of medical diagnostics, particularly due to its applications in cancer, genetic, and infectious diseases. With technological advancement, microarray has a huge potential in veterinarian diagnostics and it certainly leads to the new generation of devices such as cell biology and proteomics.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.








