INTRODUCTION
Primary pleural lymphoma is an extremely rare disorder, accounting for 2.4% of primary chest wall tumors.1,2 The clinical manifestations and characteristic pleural thickening on thoracic computed tomography are clinical clues to the diagnosis. A case of primary pleural lymphoma, diffuse large B cell type, with thickened pleura and ipsilateral pleural effusion as the initial presentation, is reported.
CASE REPORT
A 72-year-old man was referred to our hospital because of a 1-month history of persistent right chest pain. He had been in good health except for normal pressure hydrocephalus two years previously. He worked as a landscape gardener and had no illicit drug or dust exposure, including asbestos. On initial examination, he appeared well, and vital signs and physical examination were normal. Chest X-ray showed a solitary nodule measuring 15 mm in size with an extrapleural sign in the right upper lung field, as well as blunting of the right costophrenic angle (Figure 1A). Thoracic Computed Tomography (CT) confirmed the bulging nodule at the right parietal pleura and a bulky right pleural mass with a pleural effusion (Figures 1B and 1C). Both thoracic CT (Figure 1C) and Fluorodeoxyglucose (FDG) Positron Emission Tomography/Computed Tomography (PET/CT) (Figure 2) showed that the right pleural mass partially extended to the left pleura and invaded to the spinal canal through the right intervertebral foramen, which showed an intense Standardized Uptake Value (SUV) of 28.5, suggesting malignancy. Serum laboratory examinations were normal except for mild anemia, mild elevation of Lactase Dehydrogenase (LDH) (230 IU/L), Creactive protein (1.3 mg/dL), and soluble interleukin-2 receptor (956 U/mL). Furthermore, no elevation of serum tumor markers such as Carcinoembryonic antigen (CEA) (1.8 ng/mL, normal range <5.0 ng/mL), carbohydrate antigen 19-9 (5.7 U/mL, normal range < 37.0 U/mL), squamous cell carcinoma antigen (1.4 ng/mL, normal range <1.5 mg/mL), and neuron-specific enolase (8.6 ng/mL, normal range <10.0 ng/mL) were noted. Thoracentesis demonstrated elevation of the total cell count (4,200 /mL; with lymphocytes 87%, neutrophils 6%, monocytes 6%), total proteins of 4.3 g/dL, and LDH of 200 IU/L, suggesting a chronic exudative pleural effusion. The values of CEA (0.8 ng/mL), adenosine aminhydrolase (25.1 IU/L), and hyaluronic acid (43,900 ng/mL) in the pleural fluid were normal, and the cytological assessment was class II.
Figure 1: Chest X-ray shows a solitary nodule, 15 mm in size, with an extrapleural sign in the right upper lung field, together with blunting of the right costophrenic angle (A). On thoracic CT, no abnormal lesion is noted in the lung parenchyma (B), but a massive right pleural mass with pleural effusion is seen, which partially extends to the left pleura and spinal canal through the right intervertebral foramen (C).
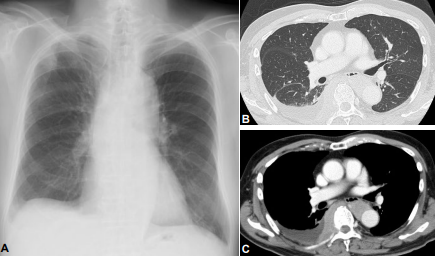
Figure 2: On FDG-PET/CT, the right pleural mass has invaded to the spinal canal and left pleura, which show intense standardized uptake values (SUVs) of 28.5. A moderate pleural effusion of the right side is also noted.
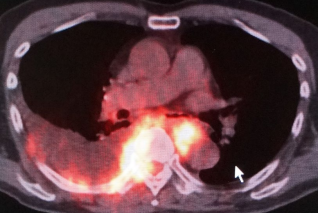
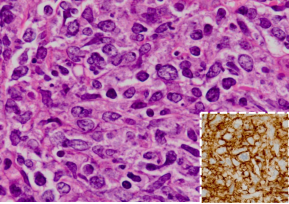
On thoracoscopy, protrusion of the surface of the parietal pleura with white colored central necrosis together with dilatation of the capillary vessels at the normal site (Figure 3) was noted. On hematoxylin and eosin staining, the diagnostic thoracoscopic pleural biopsy specimens demonstrated abundant large atypical cells (Figure 4) that were immunohistochemically positive for CD20 (Figure 4, inset), CD79a, CD10, and Bcl2, but negative for cytokeratins and calretinin.
Figure 3: On thoracoscopy, protrusion of the surface of the parietal pleura with white colored central necrosis, together with dilatation of the capillary vessels at the normal site, is noted.
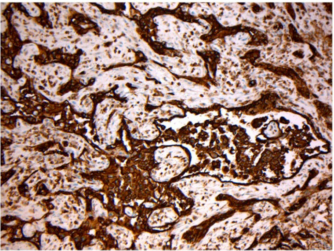
Figure 4: On hematoxylin and eosin staining, the pleural biopsy specimens demonstrate abundant large atypical cells (Figure. 4) that are immunohistochemically positive for CD20 (Figure. 4, inset)
Based on these findings, he was diagnosed with primary pleural lymphoma, diffuse large B cell lymphoma (DLBCL), Ann Arbor stage II. Thereafter, he was treated with 6 consecutive courses of R-CHOP chemotherapy (R: rituximab (375 mg/ m2 on day 1), C: cyclophosphamide (750 mg/m2 on day 2), H: doxorubicin hydrochloride (50 mg/m2 on day 2), O: vincristine sulfate (1.4 mg/m2 on day 2), and P: prednisone 100 mg/body on days 2 to 6), which resulted in complete remission, and he was discharged uneventfully.
DISCUSSION
Primary tumors account for about 5-10% of pleural neoplasms such as malignant mesothelioma, localized fibrous tumor, and pleural liposarcoma, in this order.3,4 Secondary tumors, which account for approximately 90% of all pleural neoplasms, are metastatic disease, such as lung cancer (40%), breast cancer (20%), lymphoma (10%), ovarian carcinoma, and gastric carcinoma.3,4 In this regard, although approximately 16% of patients with Non-Hodgkin’s Lymphoma (NHL) present with or subsequently develop pleural involvement during the course of disease,5 primary pleural lymphoma is an extremely rare disorder, accounting for 2.4% of primary chest wall tumors.1,2 The most frequent type of lymphoma involving the pleura is DLBCL, followed by follicular lymphoma,6 with rates of approximately 60% and 20%, respectively,7 as was the present case.
In general, clinical clues to the diagnosis of primary pulmonary lymphoma are pleural effusion, pleural thickening, and/or chest pain, as in the present case. Filly et al.8 reported that pleural effusion was seen in 7% of patients with Hodgkin’s lymphoma (HL) and 10% of patients with NHL who were previously untreated. The mechanisms of pleural effusion in primary pleural lymphoma can be due either to impaired lymphatic drainage because of mediastinal adenopathy or pleural or pulmonary infiltration, or to thoracic duct obstruction. The pleural effusion in NHL appears to be caused by impaired lymphatic drainage, and that of HL was due to direct pleural infiltration of tumor cells.9 The precise reason is unknown, but the mode of spread is rather lymphatic in HL, and contiguous and/or hematogenous in NHL,10,11 which may explain the higher affinity to parietal pleura in NHL. Similarly, though the reason is unknown, pleural involvement can be unilateral or bilateral and is more common on the left side.5
Regarding radiological findings, primary pleural lymphoma can present as an enhancing mass in the costophrenic recess with pleural effusion, corresponding to pleural thickening12,13 and/or nodular pleural infiltration, which needs differentiation from primary pleural tumors including malignant mesothelioma or other metastatic pleural diseases,14 as in the present case.
The tumor development mechanisms of malignant lymphoma arising in the pleura have not yet been established,15 but Hirai et al. demonstrated that all of nine Japanese patients with primary pleural lymphoma without a history of pyothorax had no Epstein-Barr virus (EBV) infection, as in the present case (figure not shown).
This case showed unique clinical presentation of primary pleural lymphoma in that 1) massive right pleural thickening with an ipsilateral pleural effusion, 2) no history of pyothorax associated with EBV infection. Thus, this case reminds us of the fact that we should be aware of the possibility of primary pleural lymphoma as an extremely rare tumor if the patient has pleural thickening with pleural effusion, even with no history of chronic pyothorax.









