INTRODUCTION
Fresh produce have high nutritional qualities and several health benefits attributed to them. From a nutritional point of view, fresh produce are low energy-dense foods relatively rich in vitamins, minerals and other bioactive compounds as well as being good sources of fiber.1 Consumption of fresh produce is encouraged by government health agencies, health groups and health professionals.2 Generally, fresh fruits and vegetables are believed to protect against a range of illnesses such as cancers and cardiovascular diseases.3 This campaign is helping to change dietary habits toward higher per capita consumption of fresh fruits and vegetables, particularly, in developed world. For instance the average intake of fruits and vegetables in New Zealand is nearly 400 gram per day.2 Without doubt, fresh fruits and vegetables are important components of a healthy and balanced diet, however, several new food safety issues are being associated with their consumption.4 One of the major issue is the emergence of pathogens that were not traditionally associated with raw produce. This situation has enhanced the potential for outbreaks associated with raw fruits and vegetables.
Bacterial pathogens such as Salmonella, Bacillus cereus, Escherichia coli O157:H7, Clostridium botulinum, Shigella, Staphylococcus, Vibrio cholera, Aeromonas, Campylobactor jejuni and L. monocytogenes were frequently isolated from fresh produce.5 Among all of these pathogens, L. monocytogenes is a kind of pathogenic bacterium that causes a serious disease called listeriosis. This infection has variable degree of health consequences, such as mild gastroenteritis, severe infections of the blood stream and/or the central nervous system, and even abortion for pregnant women.6 This illness is a relatively rare but has high fatality rates (30%).7 L. monocytogenes had previously been isolated from market produce i.e. cabbage, corn, lettuce, sprouts, potatoes, cucumbers, parsley, watercress and salad vegetable.8
Several outbreaks of L. monocytogenes infection associated with fresh produce have been reported in various parts of the world. Recently L. monocytogenes was responsible for the deaths of 10 people in food poisoning outbreaks of chopped celery in 2010 and 30 people due to contaminated melon in 2011.9,10 The foodborne outbreaks caused by contaminated fresh produce are predicted to increase in coming years.9 Therefore, this study was planned to investigate the status of Listeria spp. contamination in salad vegetables and selected Ready-to-Eat (RTE) food products containing fresh produce. To our knowledge this is the first investigation on Listeria spp. contamination levels in fresh produce marketed in Canterbury region, New Zealand.
MATERIAL AND METHODS
Sampling plan and samples collection
Four most commonly consumed fresh vegetables, including green cabbage, purple cabbage, lettuce, and carrot, were selected in this study. These vegetables are consumed raw or in minimally processed form. Samples of these vegetables were collected from two different fruit and vegetable markets (reported as market 1 and market 2) located in Canterbury region of New Zealand over a 5-week period. All samples were bagged separately when purchased and brought to laboratory in a way normally consumers take home. Following the raw vegetables testing, samples of 12 different RTE products that contained fresh produce as ingredient were also tested to determine possible relationship between fresh produce and Listeria spp. detection. These RTE products were also collected from two different supermarkets (supermarket 1 and supermarket 2).
Samples preparation
Briefly, 100 g of each raw vegetable sample was weighed into a stomacher bag. An appropriate amount of 0.1% peptone water was added before stomaching for 5 min. Afterwards, 1 mL of the liquid portion of stomacher contents was used to prepare serial dilutions in 0.1% peptone water. One mL of selected dilutions were transferred onto each Listeria selective agar plate (in duplicate) and incubated at 35 o C for 24 h.
Media and enumeration of Listeria spp.
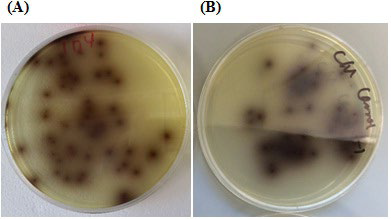
Listeria selective agar (CM0856, OXOID) was used in this study. Media plates were prepared according to manufacturer’s instructions. Typical Listeria spp. colonies were brown color. Pure Listeria monocytogenes (strain V7) culture was used as a positive control (Figure 1) and 0.1% peptone water was the negative control. Confirmation of L. monocytogenes was not carried out in this study. The numbers of typical Listeria spp. colonies were counted after incubation and reported. Colony forming units (cfu/g) were calculated using the formula:
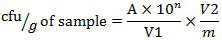
where A — number of colonies (average in two plates);
10n — level of dilution at which the counting was carried out;
V1 — volume of inoculum;
V2 — total volume of peptone water;
m — total sample weight
Figure 1: Typical L. monocytogenes colonies on Listeria selective agar (CM0856, OXOID). (A) L. monocytogenes (strain V7) pure culture colonies (B) Colonies of L. monocytogenes from a test product sample
RESULTS
Listeria spp. in selected salad vegetables
This study investigated the prevalence of Listeria spp. in four different vegetables, commonly used as salad, sampled from two fruit and vegetable markets in Canterbury region of New Zealand. All purple cabbage samples from both markets were consistently positive for Listeria spp. presence during the 5-weeks sampling period (Table1). Listeria spp. were detected in 60% and 100% of green cabbage samples from market-1 and market-2, respectively. Lettuce samples collected from both markets during week 1 to week 3 were contaminated with Listeria spp., while carrot samples had the lowest percentage (20%) of positive samples. Generally Listeria spp. was present in all vegetable types tested in this study. However, purple and green cabbage samples mostly carried Listeria spp. and the samples collected in market-2 had more number of positive samples for Listeria spp. compared to market-1. Carrots had the lowest number of samples and frequency for Listeria spp. detection.
Table 1: Comparison of Listeria spp. presence in vegetable samples collected from two different markets
|
Sampling period |
| Week 1 |
Week 2 |
Week 3 |
Week 4 |
Week 5 |
| Market-1 |
| Lettuce |
+
|
+
|
+
|
–
|
–
|
| Green Cabbage |
+
|
–
|
–
|
+
|
+
|
| Purple Cabbage |
+
|
+
|
+
|
+
|
+
|
| Carrot |
–
|
+
|
–
|
–
|
–
|
| Market-2 |
| Lettuce |
+
|
+
|
+
|
–
|
–
|
| Green Cabbage |
+
|
+
|
+
|
+
|
+
|
| Purple Cabbage |
+
|
+
|
+
|
+
|
+
|
| Carrot |
–
|
–
|
–
|
–
|
+
|
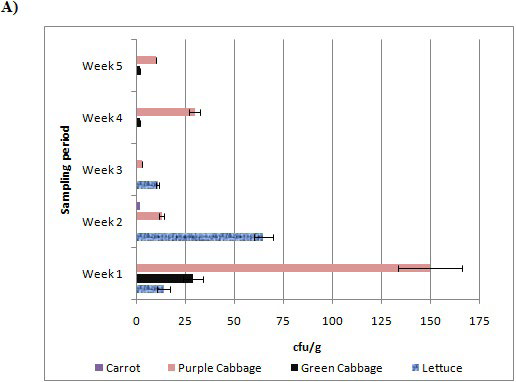
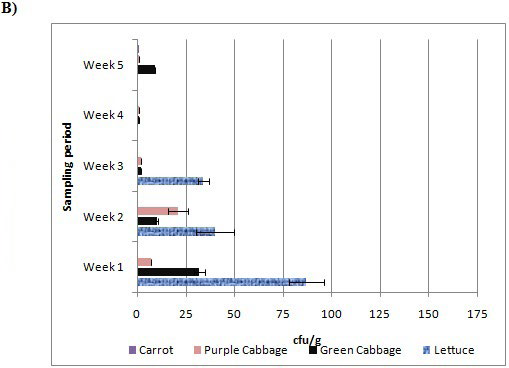
Figure 2 shows the number of colony forming units (cfu) detected in 1 g of fresh vegetable samples. Notably, in the first week, purple cabbage sample collected from market-1 had the highest number (i.e. 150 cfu/g) of Listeria spp. detected. Purple cabbage samples had more Listeria spp. counts than green cabbage and lettuce samples from market-1 in most of the cases. Lettuce samples from market-2 showed comparatively high level of Listeria spp. during the first three weeks sampling. Maximum number of Listeria spp. detected in green cabbage, lettuce and carrot were 33 cfu/g, 87 cfu/g and 2 cfu/g, respectively.
Figure 2: Total number of Listeria spp.
(cfu/g) detected in each of the vegetable sample from market-1 (A)
and market-2 (B) over 5-week period. Error bars represent standard deviations of cfu detected each week.
Listeria spp. in RTF containing fresh produce
Our curiosity to see whether Listeria spp. can be detected in RTE food products containing fresh produce led to collection of samples from two supermarkets (Table 2). Twelve samples were randomly collected once and two samples (Japanese style coleslaw and spinach and basil dip) were found in positive for Listeria spp. presence. This aspect of the study needs the detailed investigation. However, our results indicate there is potential risk associated with RTE products containing fresh produce as ingredient.
DISCUSSION
Many research reports indicated the existence of Listeria spp., L. monocytogenes in particular, on the surface of fresh vegetables. L. monocytogenes is an important pathogen and was detected in 28% of fresh vegetables positive for Listeria spp. presence.11 Althaus et al found that the samples of RTE lettuce were infected by L. monocytogenes. 12 In another report, Uzeh and Adepoju showed that L. monocytogenes was present on salad vegetables (sliced cabbage, lettuce and carrot).13 The growth of L. monocytogenes was linked to the storage condition, especially temperature. Our results also strongly suggested that L. monocytogenes was prevalent in purple cabbage, green cabbage and lettuce. These observations are consistent with previous findings mentioned above.
According to guideline for the control of L. monocytogenes in RTE products, Listeria spp. level in fresh produce is expected to be as low as possible, and the maximal safety level limit should not exceed 100 cfu/g.14 Obviously, purple cabbage from market-1 sampled in the first week had the level of Listeria spp. (150 cfu/g). The lettuce sample collected in week-1 from market-2 also found to have numbers close to maximum limits. All the other vegetable samples had detected numbers within the range of safety limits. However, we need to keep in mind that the guidelines of the food safety agencies are for regularity purpose and ambiguity of Listeria makes it more dangerous, thus Listeria guidelines need frequent review. In our opinion, it is not the number of pathogenic cells rather prevalence is more serious problem that allows this pathogen to reach to immune-compromised groups of the population (elderly, infant, pregnant women, etc).
Comparing the results of this study with Sant’Ana et al.15 who reported 7.67±0.09 cfu/g of L. monocytogenes in cabbage samples, we have detected much higher number. However, carrot samples had 6.15±0.02 cfu/g, much more than our result (i.e. 2 cfu/g). Another investigation on the risk assessment of lettuce samples from home, restaurant and farm in Korea found that average final contamination level before consumption after washing treatment in restaurant or at home were -1.50 log cfu/gor -0.146 log cfu/g, respectively. Using food safety limit for L. monocytogenes population on fresh produce (2 log cfu/g) as threshold value, contamination levels on lettuce greater than this threshold value were observed as 12.86%, 15.03% and 31.04% for farm, restaurant and home, respectively.16
Microbiological analysis of raw vegetables was followed by L. monocytogenes testing in RTE products containing fresh produce. L. monocytogenes was not present in majority of its products (Table 2). Japanese style coleslaw and spinach-basil dip collected from supermarket-1 were positive in L. monocytogenes. However, numbers were less than threshold value for RTE products. Cordano and Jacquet17 tested 154 samples of industrial minimally processed raw salads, and L. monocytogenes was not detected. However, Althaus et al12 found 5 out of 223 samples of RTE lettuce contained L. monocytogenes. Number of Listeria spp. was less than 2 log cfu/g in each sample. Data from our study and several other reports strongly suggest that Listeria spp. could pose a serious threat to the safety of fresh produce and products that contain them as an ingredient. Therefore, it is important to use appropriate measures in order to reduce or minimize contamination well.
Table 2: Listeria spp. detection in RTE food products containing fresh produce sampled from two different supermarkets.
|
Classification
|
Product name
|
Source
|
Listeria spp. detection
|
| Cole Salad |
Salad coleslaw |
Supermarket 1
|
–
|
| Coleslaw salad |
Supermarket 2
|
–
|
| Slaw |
Japanese style coleslaw |
Supermarket 1
|
+
|
| Herbslaw |
Supermarket 1
|
–
|
| Broccoslaw |
Supermarket 2
|
–
|
| Crispy salad |
Supermarket 2
|
–
|
| Dips |
Spinach and basil dip |
Supermarket 1
|
+
|
| Spinach and feta dip |
Supermarket 1
|
–
|
| Feta and spinach hummus |
Supermarket 2
|
–
|
| Cucumber and mint yoghurt dip |
Supermarket 2
|
–
|
| Burger/ Sandwiches |
Meat lover roll (burger) |
Supermarket 2
|
–
|
| Ham triple sandwiches |
Supermarket 2
|
–
|
It could be assumed that most of the products sold in supermarkets have gone through some treatments (heat or chemical) to eliminate or reduce pathogenic bacteria. Generally, the levels of L. monocytogenes could be reduced to the lowest level through different methods: 1) washing and cleaning before marketing (e.g.: making fresh produce in to salad); 2) applying a listericidal process (e.g.: pasteurization which in an effective way to reduce the presence of Listeria spp.); and 3) keeping the food in the environment which would inhibit the Listeria spp. growth.
Recently, several methods have been developed to reduce L. monocytogenes levels on the surface of fresh vegetables. For example, using balsamic vinegar from Moden approved more effective than washing in reducing pathogens including L. monocytogenes. 18 Authors also suggested that relative humidity and storage temperature also played a key role in keeping the low level of L. monocytogenes. 19 They showed that L. monocytogenes did not grow on lettuce or parsley at low temperature and a decline or stability in numbers was observed. High humidity caused L. monocytogenes to grow on cucumber epidermis and viable count increased from 4.0 to around 5.5 cfu/cm.2 It suggests dry place is more fitful storage condition for fresh produce.
In conclusion, Listeria spp. have been frequently detected in market produce such as cabbage, corn, lettuce, sprouts, potatoes, cucumbers, parsley and watercress. In this study, all four fresh vegetables (lettuce, green cabbage, purple cabbage, carrot) were found in carrying the Listeria spp. more or less. These results indicate that food safety risks are associated with fresh produce consumption. However, RTE products (containing fresh produce) are relatively safe. Further, investigations are needed to establish level of risk associated with each product type and food safety threat to consumers.








