INTRODUCTION
Mastitis, an inflammation of the mammary gland, is a complex and costly disease in dairy herds.1 Mastitis is a multi-factorial disease, as such; its incidence depends on exposure to pathogens, the effectiveness of udder defense mechanisms, the presence of environmental risk factors, as well as interactions between these factors.2 Mastitis is a global health problem for dairy animals and occurs in sporadic and epidemic forms, resulting in profound economic losses to the dairy sector in developed and developing nations.3 Mastitis could also be a danger to human health because milk from the mastitic udder of animals is contaminated with bacteria, which could be a potential source of infection for consumers,4 such as tuberculosis, streptococcal intoxication, colibacillosis, streptococcal sore throat, and brucellosis.5
Many microorganisms might cause mastitis. The majority of microorganisms that are responsible for mastitis and spoilage of milk are of bacterial origin and include Coagulase negative Staphylococcus, Staphylococcus aureus, Streptococcus agalactiae, Corynebacterium bovis, Mycoplasma species, Streptococcus uberis,6 coliforms (Escherichia coli, Klebsiella species and Enterobacter aerogenes), Serratia, Pseudomonas proteus species, environmental Streptococci, and Enterobacter species.7 Mastitis can be either clinical mastitis (CM) that gives rise to visible symptoms or subclinical. Mild CM causes flakes or clots in the milk, whereas severe cases are associated with heat, swelling, and discoloration of the udder, as well as abnormal secretion. Systemic clinical symptoms like fever, depression, and loss of appetite might occur from clinical mastitis. Mastitis can exist in the absence of visible signs of infection and is then referred to as subclinical mastitis (SCM). SCM is the most prevalent form of mastitis.8
Transmission occurs mainly at milking time through contaminated milking machines, clothes, and the hands of milkers or machine operators. Contagious mastitis is transmitted from cow to cow by pathogens for which the udder is the primary reservoir. It tends to be sub-clinical in nature. The primary reservoir of contagious pathogens is the mammary gland itself.9
The cattle population of Ethiopia is 56.71 million, which makes it the first African nation. The cow represents the biggest portion of around 20.7% of total cattle heads that are milking cows.10 However, milk production often does not satisfy the country’s requirements due to a multitude of factors. Mastitis is among the various factors contributing to reduced milk production.11 In previous studies, different prevalences of bovine mastitis were reported in several parts of our country. Accordingly, the reported prevalence was 99.9% in selected areas of southern Ethiopia,12 51.1% in the eastern part of the Amhara region,13 49.7% in Tigray, 48.5% in Bahirdar,14 74.7% around Addis Ababa,15 and 64.6% from Eastern Hararghe,3 respectively.
Moreover, mastitis affects milk quality directly through the technical characteristics and the hygienic quality of the milk and indirectly through the intrinsic milk quality. The average production loss per lactation for one infected quarter is about 1,600 pounds. The economic loss from mastitis in the urban and semiurban areas of Addis Ababa is U$58 and 78.65 per cow and per lactation, respectively.16,17 Moreover, due to its latent form, heavy financial losses and great nutritional and technological impacts can result. Because unwanted components like ions and enzymes are increased while beneficial components like lactose, fat, and casein are lowered, milk is no longer suitable for processing technology.18,19
In and around Harar, increasing population density increases the demand for milk consumption. Therefore, many farmers and business owners plant dairy farms as one investment with the aim of high milk production. In this specified study area, the disease is gaining more and more importance with the recent increase in dairy stockholders and farms. Even though there are many published papers on the prevalence and risk factors of mastitis, they still face many problems related to the disease and the increasing costs of animal feed without sufficient milk production. On the other hand, sufficient data is lacking concerning bovine mastitis, and there is also a lack of information regarding which bacterial pathogens are prevalent in the area. In addition, there is feedback indicating that commonly used drugs for mastitis treatment are less effective due to unknown reasons.20 For instance, these problems limit the willingness of farmers and farm owners. Therefore, this research is aimed at the following objectives:
• To determine the prevalence of bovine mastitis in and around Harar town.
• To assess major risk factors associated with bovine mastitis in the study area.
• To isolate major bacteria that cause bovine mastitis in the study area.
MATERIALS AND METHODS
Study Area
The study was carried out at the Harar dairy farm, which is located in Harar town and its district. Harar, the capital city of Harari Regional State, is located in the eastern part of Ethiopia at a distance of 525 km from Addis Ababa, the capital city of Ethiopia. The total geographical area of Harar town is about 343.21 km2 . It is geographically estimated at 41’59” and 58° North latitude and 9’24’10” longitude. The region shares a common boundary with the eastern zone of Oromia wereda, like Jarso wereda in the north, Fadis wereda in the south, Babile wereda in the east, and Haramaya wereda in the west. The climate of the region is one of the most pleasant in the country. The temperature is between 17.10 and 20.20 °C. The average annual intensity of precipitation ranges from 750 mm to 2000 mm. The region is mainly categorized into two agro-ecological zones, where 90% of the estimated mid-high land area is between 1400 and 2200 m above sea level, while the remaining 10% is kola 1500 m above sea level. The settlement pattern of the region is different from other regions of the country, where 62% of its population is raised in urban areas. The elevation is 1600 m to 1900 m above sea level. The urban follow morphology represents two main parts: the older Jegol and the new one.10 The farms were categorized into small-scale dairy production (SSDP), medium-scale dairy production (MSDP) and large-scale dairy production (LSDP) based on their herd size, which is less than or equal to 5, 6, up to 70, and above 70, respectively.21 The animals were kept indoors and supplemented with concentrates made from beer, molasses, and hay. Most of the farms had intensive production, in which dairy animals were kept indoors at zero grazing.
Study Design
A cross-sectional study design was conducted on lactating dairy cattle from November 2016 to April 2017 in Harar town. A crosssectional survey and questionnaire were implemented to gather all relevant data for the dairy farms based on the information obtained from the Agricultural Bureau of Harari regional state about the dairy farms.
Study Population
This study was focused on all lactating Holstein and Holstein-Zebu crossbreed cows in different age groups and parity numbers. From 33 dairy farms located in Harar city and its district, 12 dairy farms from Harar city and 5 dairy farms from its districts—a total of 17 farms having at least 15 lactating cows—were considered. In this study, dairy cattle that were kept under intensive and semi-intensive conditions were included. The herd size of the selected farms varied from 21-96 cattle, of which 15-55 were lactating cows. A total of 384 lactating cows were selected. Most of them (68.75%) were cross-breeds (Holstein-Friesian×Zebu), while a few were HolsteinFriesian (31.25%). With regard to management, 5 of the herds were managed intensively, while 12 herds were semi-intensive. The intensively managed cattle were kept indoors and received concentrate feeds in addition to hay and crop residues (such as corn stalks, wheat/barley straw, and other leftovers from grain threshing). On the other hand, the semi-intensively man-aged cattle grazed freely on pasture but received supplementary feeds in the morning and evening when they were milked. All cows were hand-milked twice daily, in the morning and evening.
Sampling Method and Sample Size Determination
A two-stage cluster sampling method was applied: at the first stage, 17 dairy farms (12 inside Harar city and 5 outside the city) from 33 dairy farms located in the Harari region were selected based on the willingness of the owner, and at the second stage, all lactating cows were selected from selected dairy farms. The sample size for the study was calculated based on the formula developed by Thrustfield22 for the random sampling method. A 5% absolute precision and a 95% confidence interval are used for determining the sample size. The sample size was determined using the formula by taking 50% of the expected prevalence.
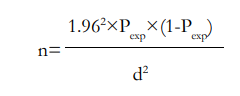
Where n is the required sample size, Pexp is the expected prevalence, and d2 is the desire for absolute precision. Based on this given formula, by substituting the given values, a total sample size of 384 lactating cows was obtained for the study population.
Study Methodology
Observation:
Data were collected by inspection of the status of the udder, housing sanitation, cow identification, place of origin, breed, age, milking system, and the like. In addition to this, the color of the milk, consistency of the milk, and content of the milk were measured.
Before a milk sample was taken from each selected cow, the udders were examined visually, followed by thorough palpation to detect possible fibrosis, cardinal signs of inflammation, visible injury, tick infestation, atrophy of the tissue, and swelling of the supra mammary lymph nodes. Rectal temperatures were taken for acute mastitis cases to check for systemic involvement of the infection. The viscosity and appearance of milk secretions from each quarter were examined for the presence of clots, flakes, blood, and water secretions.
Data about owner/farm name, cow identification, bread, age, parity, and farm hygiene were gathered by inspection of the animal and interviews with the owners. In addition to this, the color of the milk, consistency of the milk, and content of the milk were also detected. Secondary data were taken from recorded data by the Agricultural Office of the Harari region.
California mastitis test:
The California mastitis test (CMT) was conducted to diagnose the presence of subclinical mastitis, and it was carried out according to procedures given by Quinn et al.23 An appropriate amount of milk was taken from each quarter, and in each of the four shallow cups in the CMT paddle, an equal amount of the reagent (4% sodium hydroxide) was added. Then, following a gentle circular motion, positive samples showed gel formation within a few seconds.
The result was scored based on the gel formation and categorized as negative if there was no gel formation or positive if there was gel formation. If at least one quarter was positive, then the cows were taken as mastitic. Reaction was graded as 0 for negative results and +1, +2, and +3 for positive results.7
Bacteriological culture:
Milk samples were collected from all clinically infected and CMT-positive cows under aseptic conditions in sterile screw-caped bottles before the cow was treated with either an intramammary or systemic antibiotic and taken directly to the Haramaya University CVM Microbiology Laboratory, with a minimum delay for routine culture techniques. Milk samples were cultured on 5% sheep blood agar; cultures with fine bacterial growth were considered positive, and cultures with no visible growth were taken as negative.
The primary isolation or identification of bacteria was made based on colony morphology, size, and color of colonies (presence or absence), type of hemolysis, and staining reaction. To get a single purified and isolated colony, each colony having a unique character on primary culture media was taken and cultured on selective and differential media such as mannitol salt agar for Staphylococcus, Edwards’s media for Streptococcus and MacConkey agar for gram-negative bacteria. Pure isolates of E. coli were inoculated into 0.10 mL of Bile Esculin Azide (BEA). Corynebacterium species were cultured on Lysed blood agar. Furthermore, biochemical tests such as catalyst tests, coagulase tests, motility tests, and indole tests were adopted by the National Mastitis Council (NMC) in 2004.
Data Analysis
The collected data during the study periods was entered into an Microsoft (MS) Excel spreadsheet, and descriptive statistics were used to illustrate various variables in the production system, including husbandry and management variables. For data analysis, statistical package for the social sciences (SPSS) version 20 was used. The chi-square (χ2 ) test was used to assess the association among the hypothesized risk factors, namely the breed, age, hygiene, farm management system, and location, with the occurrence of the disease. In all the analyses, the confidence level was held at 95% to reveal the associated risk factors for the prevalence of mastitis.
RESULTS
The present study was conducted using a total of 384 milking cows. Out of 384 milking cows, 225 (58.6%) lactating cows were positive for mastitis, out of which 41 (10.67%) and 184 (47.9%) cows were found to have clinical and subclinical mastitis positive for the CMT, respectively (Table 1).
| Table 1. Prevalence of Clinical and Sub Clinical Mastitis at Cow and Quarter Levels |
|
Observation
|
Mastitis |
No Clinical Mastitis (%) |
No Sub Clinical Mastitis (%) |
| No Examined |
No Positive |
Prevalence
|
| At cow level |
384
|
225 |
58.6% |
41
(10.68%) |
184
(47.92%)
|
| At quarter level |
1505
|
513 |
34.1% |
131
(8.7%) |
382
(25.4%)
|
| At farm level |
17
|
16 |
94.12% |
5
(29.41%) |
11
(64.7%)
|
Out of the 1536 quarters examined, 31 (2.02%) quarters were found to be blind. On screening of the functional teats 1505 by CMT, a quarter of 131 (8.7%) and 382 (25.4%) were found to be affected by clinical and subclinical mastitis, respectively. The higher prevalence of subclinical was recorded at left rear (34.5%), followed by right front (28%), right rear (21.4%), and the least recorded was at left front (17.8%), while the highest prevalence of clinical mastitis was recorded at right front (19.4%), which is followed by right rear (8.7%), left rear (8.6%), and the least clinical score was at left front (8.5%) (Table 2).
| Table 2. Quarter Level of Prevalence of Mastitis and Blind Teats |
|
Quarter
|
CMT Positive
No (%) |
Clinical
No (%) |
Blind
No (%)
|
|
Right Front
|
105(28%) |
34(19.4) |
9(2.3%) |
|
Right rear
|
81(21.4%) |
33(8.7%) |
5(1.3%) |
| Left Front |
67(17.77%) |
32(8.488) |
7(1.86%)
|
| Left rear |
129(34.5%) |
32(8.56%) |
10(2.6%)
|
Regarding the treatment history, a higher prevalence (65.9%) was recorded at the lactating cows treated with traditional drugs, followed by 56.7% for those treated with drugs without prescriptions, and a lower prevalence (53.5%) was recorded at the lactating cows treated with professionals (p>0.05), a higher prevalence (59.9%) was also recorded on the cows kept intensively, and a lower prevalence (54.3%) on those kept semi-intensively (p>0.05). The prevalence result shows the prevalence of bovine mastitis was higher (59.9%) in those cows from Harar city and lower (54.3%) in those located in districts (Table 3).
| Table 3. The Prevalence of Mastitis within the Considered Associated Risk Factor |
|
Risk Factors
|
Categories |
Total Cows |
No. of Affected |
Prevalence (%) |
χ2 |
p value
|
|
Hygiene
|
Cross |
264 |
127 |
48.1 |
39.43 |
0.000 |
| Holstein Friesian |
120 |
98 |
81.7 |
| Poor |
67 |
55 |
82.1
|
23.82
|
0.000
|
| Medium |
224 |
127 |
56.7
|
| Good |
93 |
43 |
46.2
|
|
Parity
|
1-3 |
160 |
45 |
28.1 |
58.26 |
0.00 |
| 3-6 |
193 |
112 |
58.0
|
| 6-9 |
31 |
27 |
87.1
|
|
Age
|
4-6 |
135 |
49 |
36.3 |
64.45 |
0.000 |
| 7-10 |
167 |
103 |
61.7
|
| >10 |
82 |
73 |
89.0
|
|
Farming system
|
Intensive |
292 |
175 |
59.9 |
1.629 |
0.443 |
| Semi intensive |
92 |
50 |
54.34
|
|
Milking system
|
1 hand milking |
126 |
68 |
53.97 |
1.674 |
0.443 |
| 2 hand milking |
256 |
157 |
60.8
|
|
House type
|
Opened |
92 |
50 |
54.3 |
1.629 |
0.443 |
| Close |
292 |
175 |
59.9
|
|
Treating of infected cows
|
Traditional medicine |
33 |
19 |
65.6 |
5.475 |
0.24 |
| By professional |
200 |
107 |
53.5
|
| Drug without prescription |
151 |
99 |
57.6
|
From 513 cultured samples, 341 (66.5%) samples showed growth on 7% sheep blood agar and were positive for bacteria. The isolated bacterial species were central nervous system (CNS) 97 (28.5%), S. aureus 73 (21.4%), Streptococcus species 94 (27.5%), E. coli 49 (14.4%), Micrococcus species 18 (5.3%), and C. species 10 (2.9%) (Table 4).
| Table 4. Frequency Distribution with Percentage Prevalence of Various Species of Bacteria |
|
Species of Bacteria (%)
|
Frequency |
Prevalence
|
| Coagulase Negative Staph |
97
|
28.5%
|
| S. aureus |
73
|
21.4%
|
| Streptococcus species |
94
|
27.5%
|
| E.coli |
49
|
14.4%
|
| Micrococcus species |
18
|
5.3%
|
| Corynebacterium species |
10
|
2.9%
|
| Total |
341
|
66.5%
|
DISCUSSION
The present study was conducted on 384 lactating dairy cattle through a combination of the CMT, an examination of clinically infected cattle, and a questionnaire survey to determine the prevalence of bovine mastitis, isolate major bacteria, and check different risk factors for bovine mastitis in Harar city and its districts. In the present study, the overall prevalence of mastitis was 58.6%, which agrees with the previous findings of Bedane et al24 Haftu et al25 and Benhamed et al26 who recorded 59.1% in Borena, 57.5% in West Algeria, and 56.16% in West Algeria, respectively.
The overall prevalence of 58.6% reported in the present study was in close agreement with the results of various researchers in different corners of the region, like Lakew et al,27 Sori et al9 and Getaneh,28 who recorded 64.6% from Assela, 52.8% from Sebeta and 63.02% from Bahirdar, respectively. However, the overall prevalence reported in the present study was lower than the previous findings of Mekibib et al29 and Zeryehun et al,15 who reported 70.1% from Holeta Town and 74.7% around Addis Ababa, respectively. The finding was higher than previous reports of mastitis in some parts of Ethiopia, Kerro et al,12 Biressaw et al30 and Mungube et al16 who reported 40.4% in southern Ethiopia, 42% in Arsi Bilbilo district, and 46.6% from the central highlands of Ethiopia, respectively. This variation may be due to mastitis, a complex disease involving interactions of various factors such as management and husbandry, environmental conditions, animal risk factors, and causative agents.5
The prevalence of subclinical mastitis reported during the study was 47.9% at cow level, which was higher than the result of clinical mastitis, 10.7%. This closely agrees with the previous prevalence reported by Sanotharan et al31 and Mekbib et al,29 who reported 43.4% from Sri Lanka and 48.6% from Holeta Town. On the other hand, it is lower than the findings of Getaneh28 and Bedada et al,32 who reported 59.38 and 55.8%, respectively. Most of the time, when comparing clinical and sub-clinical mastitis, clinical mastitis is lower than that of sub-clinical mastitis, and this is because the treatment of clinical mastitis is commonly practiced.33 And this could also be attributed to the little attention given to subclinical mastitis while treating clinical cases. Farmers in Ethiopia are not well-informed about the silent cases of mastitis.34
The prevalence of subclinical mastitis in this study is relatively higher than the previous reports by Mekebib et al29 and Girma,18 who reported prevalences of 34.8% and 34.4%, respectively. Because mastitis is a complex disease involving the interactions of several factors, mainly management, environment, and factors relating to animals and causative organisms, its prevalence is expected to vary from place to place. Since environmental factors play a significant role, the prevalence of subclinical mastitis varies in dairy animals.5
The clinical mastitis prevalence in this study was 10.7%, which was comparable with that of Zeryehun et al3 from Eastern Hararghe, Tesfaye,33 who reported 7.3% in Adama and 11.9% in Bahir Dar and its surroundings Bitew et al,35 Bedada et al,32 who reported a prevalence rate of 10.3%, on the other hand, it was higher than the findings of Adugna,36 who reported 5.7% in Dire Dawa and Haramaya University Dairy Farm, Belachew,37 and Gizat,14 who reported 0% in local Zebu lactating cows in and around Bahir Dar and 0.7% from Debrezeit, respectively. This difference is due to differences in hygiene, breed, and the lack of a veterinary clinic in the study area.
The quarter prevalence of mastitis (34.1%) found in this study was comparable with the finding of Bachaya et al38 in Pakistan, who reported 35.25%. On the other hand, it was lower than the findings of 57.1% at Zeryehun et al3 from Eastern Hararghe and 62.3% at Addis Ababa, Zeryehun et al.15 This could be due to differences in breed, milking system, farming system, poor hygiene, and management system. With regard to the prevalence of mastitis in each quarter of the udder, the right front quarters were affected with the highest infection rate (47%), while the left hindquarters are the second with a record of 43%. I disagree with the finding of 50.5% releasing hormone (RH) and 43.9% luteinizing hormone (LH) by Zeryehun et al.3 The highest infection rates recorded by this study in the right front are due to the primary exposure of this teat to poor hygienic milker hands, while the high prevalence of subclinical mastitis in the left hind quarter (34.5%) is due to the production capacity of the rear quarters, the high chance of getting fecal and environmental contamination, and the incomplete milking of this teat since it milked last most of the time.
The prevalence of blindness in this work is higher when compared with that reported by Haftu et al39 and Bitew et al35. The blind quarters observed in this study are an indication of a serious mastitis problem on the farms and the absence of culling that should have served to remove a source of mammary pathogens for the cows.
The association of mastitis occurrence with parity was evaluated and found statistically significant at the 5% level of significance. The findings reported in the current study are supported by the previous reports Mekibib et al,29 Enquebahir et al,39 Tamirat40. This is attributed to the increased opportunity for infection with time and the prolonged duration of infection, especially in a herd without a mastitis control program.5 The prevalence of mastitis was 81.7% in Holstein and 48.1% in cross breed cattle, indicating a significantly higher prevalence of mastitis in high milkyielding cows (p<0.05).
The prevalence of CMT positivity recorded in the present study for intensive systems (59.9%) and semi-intensive systems (54.43%) was closely related to the findings of Sanotharan et al,31 who recorded 66.7% and 44.3% for intensive and semi-intensive systems in Sri Lanka, respectively. A high prevalence was observed in the intensive system, and the lowest prevalence was found in the semi-intensive system. These differences in prevalence rates of subclinical mastitis might be due to differences in breeds of animals, management practices, treatment histories, milking systems, and the tests used for screening the milk samples.
The result showed that cows in closed housing systems had a higher prevalence (59.9%) of CMT positivity than those in open housing systems (54.43%). It was comparable with the findings of Sanotharan et al,31 who recorded 56.8% and 13.3% for closed and open houses, respectively. Closed housing systems increase the risk of mastitis because the confinement of the animals and the contagious microorganisms in the various litters increase the chance of mastitis.41
The result showed a higher prevalence of mastitis occurrence in cows milked with two hands (60.8%) and a low prevalence in cows milked with one hand (50.3%). This may be due to the higher exposure of teat milked with two hands to mastitis-causing pathogens than cow milked with one hand. The prevalence of mastitis was significantly (p≤0.05) associated with milking hygiene practice. The results revealed that animals with poor hygiene during the milking process had a high prevalence of mastitis. Poor hygiene of the milking process was also identified as a risk factor for the occurrence of bovine mastitis in another study in Ethiopia.42
The result showed a higher prevalence of mastitis occurrence in cows treated with traditional medicine, followed by those treated by drugs without a prescription, and a lower prevalence in those treated by professions (65.6%, 57.6% and 53.5%). This may be due to the formation of drug resistance by mastitis-causing pathogens.
The present study resulted in the isolation of numerous pathogenic bacteria. The most dominant pathogenic species that cause clinical and subclinical mastitis in the study area were coagulase-negative Staphylococcus (28.5%), followed by S. species (27.4%), and S. aureus (21.4%), which is almost similar to the findings of Zeryehun et al3 from the Eastern Hararghe Zone. In this study, the least isolated bacteria were M. species (5.3%) and C. species (2.9%), which is in agreement with reports by Sori et al.43
The commonly isolated genera of bacteria in the present study (Staphylococcus, Streptococcus, and Escherichia) agree with the findings of Abdurrahman,42 Kalla et al44 and Abera et al,45 who noted Staphylococcus, Streptococcus, and Escherichia as major mastitogens.5 It was asserted that S. aureus is well-adapted to survive in the udder and usually establishes a mild subclinical infection of long duration, from which it sheds in milk, facilitating transmission to healthy animals, mainly during milking procedures.
S. species were also prevalent, accounting for 27.5% of the total isolates. The finding in the present study is in agreement with that noted by Hawari et al,46 who reported a 26.2% relative frequency of S. species in Jordan, and Atyabi et al,47 who found 33.54% at farms around Tehran. However, it is higher than the finding of Bitew et al35 at Bahir Dar and its environs (13.9%).
E. coli occurred in 14.4% of the isolates. The finding was comparable with the report of Hawari et al46 in Jordan (15.6%). However, it was greater than the previous reports by Mekibib et al29 at Holeta (4.6%) and Sori et al9 in and around Sebeta (0.75%).
CONCLUSION
This study revealed that mastitis is the major problem with lactating cows in the dairy farms of Harar city. Moreover, this study found the prevalence of mastitis at the cow level (58.6%), and at the cow level, the prevalence of clinical and subclinical mastitis is 10.68% and 47.92%, respectively, as well as at farm levels (94.12%). Subclinical mastitis was the major type of mastitis in the area. This study found cows on dairy farms are maintained in an inadequately hygienic environment, with poor animal health services and a lack of proper attention to the health of the mammary gland, which are determiners of the occurrence of mastitis. Coagulase Negative Staphylococcus and S. aureus were also the dominant bacterial isolates in the study area. From 513 cultured samples, 341 (66.5%) samples showed growth on 7% sheep blood agar and were positive for bacteria. The isolated bacterial species were CNS 97 (28.5%), S. aureus 73 (21.4%), S. species 94 (27.5%), E. coli 49 (14.4%), M. species 18 (5.3%), and C. species 10 (2.9%).
RECOMMENDATIONS
• Attention should be given to the informal use of drugs to reduce drug resistance.
• Regular training for milkers and dairy keepers on the risk of mastitis.
• Proper hygiene of the milker’s hand and teat should be needed before and after milking.
• Available space should be needed for those cows kept in intensive and closed houses to reduce the risk of contagious mastitis.
• Thorough screening of high-producing and old-aged cows should be needed further research is needed to identify other risk factors for mastitis.
ETHICAL APPROVAL
The Haramaya University College of Agricultural Science Animal Research Ethics Review Committee ruled that no formal ethics approval was needed to conduct this study because there were no invasive procedures or experiments to be conducted and no risk of harm to the research subjects involved. Before the study, informed consent was obtained from the owners and/or managers of the dairy farms included. Best-practice veterinary care was utilized in this research, and all procedures followed the proper guidelines and regulations.
ACKNOWLEDGEMENTS
The authors express special thanks to the owners or managers of the dairy farms included in the study for their collaboration during the study period. Members of the veterinary departments in the surveyed districts are also very much appreciated for their cooperation in the field. The authors are thankful to the editor and an anonymous reviewer for their constructive comments that helped improve the quality of this paper.
AUTHOR CONTRIBUTION
All authors took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.