INTRODUCTION
Tissue damage resulting from traumatic injury or surgical procedures activates signaling cascades that lead to the transmission of pain signals. Avoidance of pain-related morbidities is necessary for recovery and rehabilitation after surgery or traumatic insult. Aside from unnecessary patient suffering, inadequate pain control results in unchecked sympathetic outflow, which has detrimental multi-systemic physiologic sequelae. In the cardiovascular system, poorly managed pain results in hypertension and tachycardia, increasing myocardial oxygen demand and placing the patient at risk for cardiac ischemia or myocardial infarction.1,2 Elsewhere, vasoconstriction decreases flow to the surgical site, increasing risk of surgical site infection. In the limbs, vasoconstriction leads to venous stasis, increasing the risk for thromboembolic events.1,2 The sympathetic response can also lead to urinary retention, respiratory compromise, catabolic stress, inflammation, immunosuppression, sleep disturbance, and postoperative ileus. These comorbidities can prolong hospital stay and increase health care expenditures.
Adequate pain control helps facilitate recovery. Preventing pain related comorbidities allows for early ambulation, faster return to bowel motility, and increased patient satisfaction.2 Pain control regimens can have adverse effects of their own.3 Opioid therapy, the historical mainstay of pain management, can result in respiratory depression, nausea, constipation, postoperative ileus, and pruritus. While opioids are effective analgesics, their use as a sole analgesic may lead to adverse events, further complicating recovery and prolonging length of hospital stay. Other analgesics are not without their own intrinsic risks. NSAIDs can result in renal compromise, surgical bleeding, decreased bone healing, GI bleeding, and cardiovascular events.3 Gabapentin use can result in heavy sedation and dizziness.3 Local anesthetics can cause toxicity, seizures, and cardiovascular compromise. Therefore a balanced approach to pain management is necessary for patient safety and satisfaction.
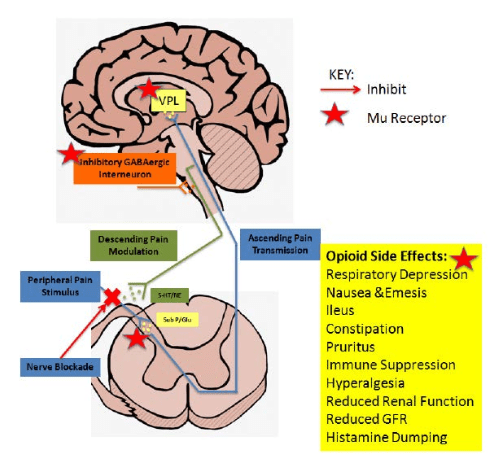
Multimodal therapy for pain management includes an array of techniques and treatments, not limited to neuraxial analgesia with single shot or continuous epidural, single shot or continuous Peripheral Nerve Blocks (PNB), opioids, acetaminophen, anti-inflammatory agents, anticonvulsants, NMDA inhibitors, antidepressants, and anxiolytics.4 Multimodal therapy targets many different pathways of pain transmission.4 As seen in Figure 1, opioids are able to modulate pain in one pathway of the central nervous system at the level of hypothalamic pituitary functions. However, pain is transmitted via multiple pathways in different parts of the body, both centrally and peripherally. Multimodal therapy will prevent pain transmission in several pathways in order to reduce inflammation, catabolic stress, and recovery time for patients.
Figure 1: Modulatory effect of morphine on pain. Morphine is able to cross the blood brain barrier and enter the CNS. Once in the CNS morphine binds to μ receptors to inhibit pain transmission. This occurs in the dorsal horn of the spinal cord stopping glutamate and substance P release, in the VPL of the thalamus and in the descending pathway on GABAergic interneurons, which allows the descending pathway to inhibit pain sensation.
An increasingly utilized addition to a balanced multimodal perioperative pain management strategy is the use of regional anesthetic techniques.5 While epidurals and intrathecal injections with local anesthetics and adjuvants have been shown to dampen the surgical stress response, the evidence for peripheral nerve blockade is still being gathered. With the advances in the placement of Peripheral Nerve Catheters (PNC) using ultrasound guidance, PNCs are becoming more widely utilized.6 This review will focus on the benefits of PNCs in terms of decreased opioid consumption and decreased physiologic response to pain. It will also discuss how this translates to shortened hospital stay and patient satisfaction.
PERIPHERAL NERVE BLOCKADE (PNB)
The utilization of peripheral nerve blocks (PNBs) for postoperative analgesia has increased with advances in block placement techniques. In the past, peripheral nerve blockade was achieved with needle placement by anatomic landmarks and nerve localization was accomplished with elicitation of paresthesia. After the emergence of electrical stimulation, nerves could be localized without the elicitation of painful stimuli; however nerve block success was inconsistent, limiting widespread implementation of nerve blockade in postoperative pain management.6
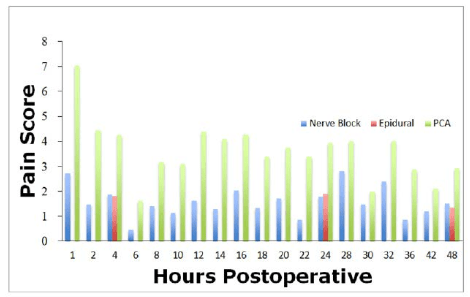
The advent of ultrasound guided regional anesthesia allowed for the visualization target nerves with the placement of local anesthetics and catheters in close proximity to these target nerves.6 As techniques using ultrasound for placement have evolved, both single shot and continuous PNBs have become more widely utilized. With continuous PNBs patients are able to receive a continuous infusion of local anesthetic around the nerve or plexus. Accordingly, continuous PNBs compared to single PNBs were associated with decreased pain ratings on postoperative days 0,1, and 2; decreased overall opioid use; decreased nausea; and higher patient satisfaction scores compared to single- and multiple-injection techniques (Figure 2).7,8 Continuous PNBs give physicians the flexibility to adapt to the differing pain thresholds of patients by dropping the volume or concentration of local anesthetic on initial dosing, which decreases the risk of systemic toxicity. Furthermore, by reducing the large initial doses, one is reducing the likelihood of motor and sensory blocks that have been associated with falls and positional injuries.9,10
Figure 2: Compilation of data from published reports showing the difference in pain scores postoperatively of nerve blocks, epidurals, and PCA analgesia. PCA has much higher pain scores on average when compared with nerve blocks and epidural analgesia.
OPIOID PATIENT CONTROLLED ANALGESIA (PCA)
Opioids are able to modulate pain in the central nervous system by blocking pain transmission (Figure 1). A variety of adverse side effects are associated with opioid analgesia, including respiratory depression, postoperative ileus, constipation, emesis, inhibition of cough reflex, drowsiness, reduced glomerular filtration rate, reduced renal function, tolerance, opioid hyperalgesia, immune suppression, and non-allergic histamine dumping from mast cells.11 Although PCA has been shown to be an effective method of inducing analgesia, research in pain management and analgesic techniques suggests that, as a sole modality, PCA is less efficacious than other analgesic techniques. Although use of PCA is widespread, analgesic strategies are shifting away from a single modality approach toward a multimodal pain management approach.
PERIPHERAL NERVE BLOCKADE AND NARCOTIC CONSUMPTION
Side-effects associated with opioid pain relief contribute significantly to the cost of postoperative care. This section will focus on the decrease in narcotic consumption that occurs when PNBs are used to provide postoperative analgesia.
Narcotic consumption is decreased when other analgesic modalities are added to a postoperative regimen. A review of narcotic consumption in patients who received single shot PNBs compared with Continuous Peripheral Nerve Blocks (CPNBs) revealed that CPNBs were associated with decreased maximum pain scores on postoperative days 0, 1, and 2 (Figure 2).7 There was also an overall decrease in narcotic consumption in patients with CPNBs compared to patients receiving a single shot PNB. The opioid sparing in the CPNB group lead to decreased incidence of nausea and other opioid-related side-effects.3
Similarly, Edwards et al12 showed that CPNBs could decrease opioid consumption on postoperative days 0, 1, and 2 compared to single shot PNBs in patients who underwent Total Knee Arthroplasty (TKA). Indeed, PNBs protect against opioid-induced cardiovascular, respiratory, and gastrointestinal side effects.8,12,13 Although continuous PNBs have been shown to be more efficacious in treating postoperative pain9,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 and decreasing opioid use, single injection PNBs have been demonstrated to provide superior pain control and decreased side-effects compared to opioid monotherapy.8,13
Altering the concentration of the local anesthetic used in the block can also modify narcotic consumption. Aguirre et al9 increased the concentration of ropivacaine in interscalene blocks for rotator cuff repairs and found significant reductions in morphine consumption. Likewise, they saw decreased nausea, constipation, improved sleep patterns, and increased patient satisfaction.9 A meta-analysis study in 2006 concluded that CPNBs provide superior pain control compared with opioids.8 The study revealed significantly lower pain scores at multiple time points in patients with CPNBs.8 The authors concluded that CPNBs can decrease health care expenses by reducing narcotic-related side-effects.8
Edkin et al14 performed a prospective study on femoral nerve blocks as an substitute to parenteral narcotics for analgesia after anterior cruciate ligament reconstruction. The Winnie 3-in-1 femoral nerve block technique was used for analgesia. The results showed that of the 24 patients who received the nerve block, 92% did not take any parenteral narcotic.14 The study showed significant reductions in the patients’ narcotic requirements and an equally significant extension of the time between incision and the patients’ initial narcotic dose.
In another prospective study, Borgeat et al23 investigated the difference between patients receiving Patient-Controlled Interscalene Anesthesia (PCIA) via continuous infusion of 0.2% ropivacaine and opioid Patient-Controlled Anesthesia (PCA) via infusion of IV nicomorphine. The patients receiving PCIA had significantly better pain scores in the 12-48 hour period after the procedure.23 They also noted that nausea and pruritus occurred significantly more in the PCA group.23 Although this study did not evaluate how PNBs decrease the use of narcotics in the healthcare system, it did show that PNBs are an efficacious form of analgesia that can lead to decreased side-effects and remove the potential for narcotics dependence.
Two studies done by Chelly et al16 and Singelyn et al29 compared postoperative opioid consumption in patients undergoing TKA with either PNBs, PCA, or epidural analgesia. Chelly et al16 found that using PNBs reduced the morphine requirement postoperatively by 74% compared to PCA and by 35% when compared with epidural anesthesia. It was also reported that PNBs provided better recovery and decreased side-effects as compared to epidurals and PCAs.16 Patients receiving PNBs were also found to report less severe maximal pain scores.16 Singelyn et al.29 found that the use of morphine postoperatively did not differ between the PNB and Epidural group. There was a significant difference between the PCA and PNB/Epidural use of morphine: PNB/Epidural groups used much less morphine. Although there was no significant difference in morphine usage between epidural and PNB, there was a significant difference in the incidence of side-effects: those in the PNB group had a much lower incidence than the epidural group.29
Lastly, a group of studies done by White et al in 2003, Ilfeld et al in 2002, and Siddiqui et al in 2007 compared the use of PNB to a saline control. Each patient received a similar anesthetic for surgery, however the trial group received a PNB during the surgery, and the control group received saline. Although the studies varied and sets of patients were tested using different procedures, they agreed that, compared to saline, PNBs used in conjunction with local anesthetics provided significantly better pain relief and decreased postoperative narcotics use. 9,18,19 Ilfeld et al9 noted that the PNB provided such powerful analgesia that 80% of the patients receiving the local anesthetic did not require a single opioid tablet during their infusion. More specifically, these patients had an average resting pain of less than 1 on a 0-10 scale. Siddiqui et al. also noted that, like prior studies, there was less vomiting, nausea and respiratory depression in the trial group than the control group.19
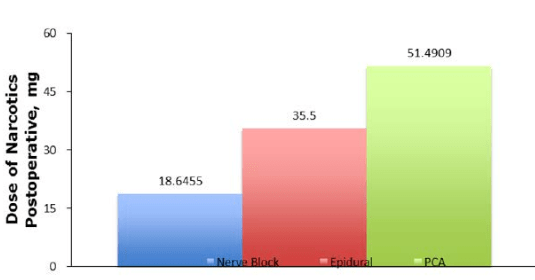
Although not all literature agrees that PNBs can reduce pain and narcotic use,30 most of the literature has shown that PNBs have the ability to reduce postoperative narcotic consumption (Figure 3), as well as give better maximal pain scores for patients postoperatively. Not only can PNBs decrease health care expenditures, they may also reduce the prevalence of narcotic dependence. PNBs, further, are advantageous because they provide an avenue by which the comorbidities associated with opioid use can be avoided.
Figure 3: Compilation of data from the literature comparing the average amount of narcotics used postoperatively between nerve blocks, epidurals and PCA. That data shows that when comparing to PCA,
both epidural and nerve blocks have lower amounts consumed narcotics postoperatively.
INFLAMMATORY RESPONSE
Inflammation after surgery is a major contributor to postoperative pain. Pro-inflammatory cytokines are involved at the site of inflammation, in the dorsal root ganglion, and within the spinal cord, resulting in neurogenic pain.31,32,33,34,35 After a tissue stressor, such as surgical insult or trauma, leukocytes are activated and migrate into the circulation. These leukocytes gather at the site of inflammation, causing the release of cytokines, resulting in a more robust local immune responss.36 Surgical trauma has been shown to provoke an inflammatory state that causes the release of inflammatory cytokines. This cytokine release results in inflammation at the sight of injury as well as inflammation that cause systemic effects. Such systemic effects are tachycardia, tachypnea, leukocytosis, and pyrexia.37 Prostaglandin E2, COX-2, and cytokines such as IL-1β, IL-6, and TNFα induce nociception by stimulating the free nerve endings of small fiber axons (A delta and C fibers) causing hyperalgesia and allodynia.20,31,33,37,38,39,40,41,42,43 Tissue stressors, such as surgery or trauma, provoke a neuroendocrine stress response resulting in local and systemic inflammation. The neuroendocrine stress response is regulated via the hypothalamic pituitary axis, adreno-medullary axis, and the parasympathetic nervous system. Similarly, synthesis of acute phase proteins produced in the liver also play a role in the stress response, as well as the modulation and release of inflammatory cytokines.36 Reducing the inflammatory response can lead to decreased immunosuppression which enhances recovery.36,44 Release of substance P and neurokinin have been shown in murine models to induce peripheral inflammation. This increase in peripheral inflammation led to a quick rise in axonal transport of the neurogenic substances (substance P and neurokinin) that peaked on the first day after insult, and subsequently returned to normal one week after insult.45,46 The initial activation of inflammation in the periphery results in the activation of the systemic responses. Administration of anesthetics surrounding the nerve or tissue near the initial site of inflammation halts the development of swelling and reduces systemic effects.47
Using a knee arthroplasty model, Bagry et al36 studied the effect of a continuous PNB on the inflammatory response. Specifically, they compared PNB to the patient-controlled analgesia inflammatory response. These researchers measured plasma glucose, serum insulin, serum cortisol, C-reactive protein, leukocytes, and interleukin-6 at 3, 8, 24, and 48 hours after surgery. Bagry et al36 observed no difference in glucose, insulin, and cortisol levels measured between the two groups. C reactive protein and leukocyte counts were reduced in the patients receiving a nerve block. These results showed a correlation between maximal leukocyte count and inflammatory mediators IL-6 and CRP was identified.39 This research suggests that PNBs are able to suppress the inflammatory response by decreasing CRP and leukocytes. Since leukocytes were correlated with decreases in IL-6 and CRP, it may be possible that PNBs are able to repress the body’s cytokine-mediated inflammatory response.
Deruddre et al used a murine model to gauge the effect of nerve blocks on the axonal transport of TNFα, edema, and TNFα receptor expression. Using carrageenan injections to induce inflammation, the study revealed that, relative to mice without nerve blocks, those with nerve block had markedly decreased edema after injection.48 To study the influence of PNBs on axonal transport, they used five study groups: one received a saline nerve block (control), the second received drug-free microspheres (control), the third received bupivacaine, the fourth received colchicine, and the fifth received an epinephrine block. During the 36-hour study period a significant decrease in the transport of TNFα occurred when comparing the controls to the bupivacaine nerve block. The colchicine group had levels of TNFα transport that were statistically different from controls; however this difference only lasted during the first 24 hours.48 By hour 30, the difference was gone.48 In the three hours after carrageenan injection, there was a significant increase in TNFα-receptor 1 levels which plateaued at the 3 hour mark. When treated with bupivacaine, the TNFα-receptor 1 levels maintained their basal concentration.48 This concentration differed significantly from the control mice.48 TNFα-receptor 2 levels were also found to have low concentrations at baseline. Additionally, these levels did not increase when inflammation was induced with carrageenan.48 This seems to evidence clinical value in using PNBs as in this model it was able to reduce both edema and transport of inflammatory mediators at the axonal level.
Studies by Beloeil et al49,50 in 2006 and 2009 showed that nerve blocks performed with bupivacaine reduce thermal hyperalgesia, mechanical hyperalgesia, edema, and markers of inflammation. Using injections of carrageenan to induce inflammation in mice, they investigated the formation of edema, thermal nociceptive withdrawal, production of PGE2, and COX1/2 expression in the spinal cord and dorsal root ganglion.50 It was observed that bupivacaine significantly decreased edema.38,50 When comparing thermal nociceptive withdrawal reflex in the mice with and without the block, they noticed significant hyperalgesia in those treated with carrageenan without a nerve block.50 The study also showeda significant PGE2 increase in subjects that received carrageenan without nerve block. When carrageenan was injected after the nerve block was placed, the levels of PGE2 did not vary significantly from the basal levels.50 There were also marked differences in COX1 and COX2 expression. In control mice, COX1 was expressed at low levels and COX2 was almost completely absent in the spinal cord and dorsal root ganglion.38 Throughout the entire experiment, COX1 levels were unchanged the entire time.50,51,52,53 COX2 mRNA levels were increased in mice treated with carrageenan that did not receive nerve block.50,52,53 The COX2 levels significantly increased throughout the spinal cord (both sides) but only increased on the side of injection at the dorsal root ganglion.50 Animal models showed that when given a bupivacaine block, levels of COX-2 mRNA was impaired. This impairment was seen on the same side of the dorsal root ganglion. This was significantly less compared with the non-block group. Furthermore, the bupivacaine block was able to impair contralateral COX-2 expression in the dorsal root ganglion.
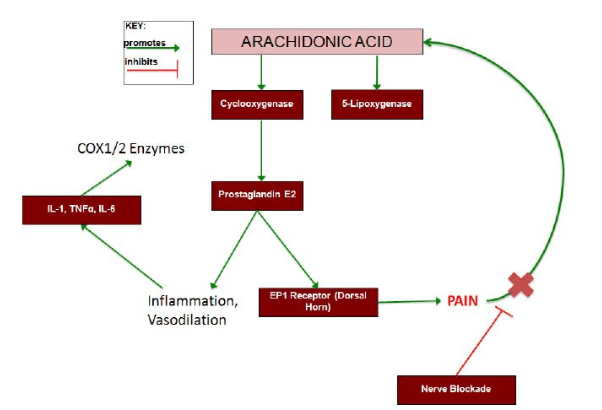
Figure 4 shows how the inflammatory markers interact with each other when causing pain. Nerve blockades in the murine models were shown to decrease levels of TNFα as well as COX1 expression. PNB’s are known to stop the pain transmission, which has a regulatory affect on the arachidonic acid pathway. The findings in the studies mentioned above have been promising. By seeing decreases in inflammatory cytokines after administration of PNBs gives hope that these anesthetic methods may help dampen the inflammatory response and speed up recovery time. However, current research involving these inflammatory markers in human subjects is lacking. Investigating similar processes in human subjects would be beneficial for understanding how regional anesthesia can dampen the inflammatory response.
Figure 4: Regulation of pain by arachidonic acid pathway. This schematic diagram shows how arachidonic acid pathway could further modulate the pain response. Cyclooxygenase (COX) converts arachidonic acid into prostaglandin E2, which causes inflammation and vasodilation leading to inflammatory cytokine release. Inflammatory
cytokines, IL-1, TNFα, and IL-6, can up-regulate the activity of the COX1/2 enzymes further producing PGE2. PGE2 is also sensed by EP1 receptors in the dorsal horn causing pain, which modulates the activity of the AA pathway.
POST OPERATIVE HORMONAL STRESS RESPONSE
Pain directly triggers hormonal stress responses. During surgery or traumatic injury, tissue destruction results in local and systemic release of signaling molecules that activate a neuroendocrine and inflammatory response. A number of systems are affected resulting in significant pituitary hormone secretion, insulin resistance, cytokine production, neutrophil leukocytosis, lymphocyte proliferation, and other responses.54,55,56 The severity of a patient’s stress response to surgery depends on a variety of factors. A patient’s age, the severity of pain or trauma, the location of the injury, and the type of anesthesia they receive affects the management of surgery and or pain.61,62 These hormonal responses are mediated by afferent nerve pathways and the central nervous system and they are linked via complex signaling networks.62
Opioids are known to suppress the secretion of hypothalamic and pituitary hormone during general anesthesia.56 McDonal et al57 demonstrated this by looking at morphine’s effect on the hypothalamus. Patients who received morphine showed suppression of corticotrophin release, which resulted lower cortisol levels. Similarly, morphine has also been shown to inhibit cortisol release in cardiac bypass patients and during upper and lower abdominal surgeries.58,59,60
Regional anesthetic techniques, such as epidural anesthesia, can inhibit endocrine and metabolic responses in surgeries taking place in the lower half of the body. Epidural blockade of T4-S5 preoperatively prevents cortisol release and modulates the hormonal response.54 Epidurals can block both the hypothalamic pituitary axis and the efferent autonomic pathways to both the liver and the adrenal medulla. This inhibits the hormonal responses from both organs, stopping the hormonal stress response from being activated.54
Studies have shown that different anesthetic techniques have different affects on the body’s hormonal reaction to pain and surgical stimuli. Celic-Spuzic et al61 showed that the use of epidural anesthesia lowered the peak levels of cortisol during and after surgery when compared with patients who underwent general anesthesia. It was also shown that levels of ACTH, which accelerates the rate of catecholamine release, were much lower in patients who underwent regional techniques when compared with general anesthesia patients. Similarly, Breslow et al63 showed that patients with regional anesthesia had lower levels of cortisol and norepinephrine release both during surgery and postoperatively (compared with those who underwent general anesthesia).25 Pflug and Halter64 compared the effects of spinal and inhalation anesthesia. Their results showed that spinal anesthesia dampened the endocrine response and the adrenergic tone. They noted suppression in norepinephrine, epinephrine, and cortisol for patients with spinal anesthesia compared with inhalational anesthesia. Similarly, Soto et al65 showed that intraoperative levels of cortisol, noradrenaline and total catecholamine levels were significantly lower when an epidural anesthetic was used compared to a general anesthetic.
Reducing the body’s response to pain and stress, although a useful way to hasten recovery may place the patients at risk of becoming immunocompromised or decrease their capability to respond to stress. The benefits of using regional anesthesia and general anesthesia still outweigh possible side-effects. Literature has shown that, compared to spinal anesthesia, the use of general anesthetics maintains less desirable levels of catecholamines, epinephrine, norepinephrine, and cortisol in the plasma.2 General anesthesia, however, is still able to diminish the surgical stress response via the opioid pathway.2
Further studies are needed to determine how peripheral nerve blockade can be employed to further suppress the surgical stress response during the postoperative period.
HOSPITAL LENGTH OF STAY
Turker et al66 showed that, when comparing PNB to epidurals, nerve blocks provided decreased motor block and limb anesthesia. Although negative side effects of PNB’s exist, including motor loss, anesthesia of limbs, and anesthetic toxicity10,67 these side effects can be managed through careful titration of local anesthetic infusions.
PNBs have been shown to reduce postoperative narcotic consumption and can help to provide sustained analgesia for patients after major surgery. Together these variables have a significant impact on the length of hospital stay. Several studies have shown that postoperative pain control is an important determinant of hospital length of stay.7,14,15,16 Chelly et al.16 investigated differences in outcomes based on postoperative pain control modalities. In comparison to epidural analgesia or CPNB, patients receiving morphine PCA had an increase in respiratory depression, constipation, pruritus and sedation, which led to a prolonged hospital stay. Likewise, Bingham et al7 also showed that, when coupled with rehabilitation, CPNBs facilitate early hospital discharge. In another study, Edkin et al14 showed that the use of CPNBs facilitates same-day discharge for several surgery types that would normally require overnight admission for pain control. The average time of hospitalization was significantly decreased for patients using femoral nerve blocks as a means of analgesia, instead of morphine PCA. In results from patient questionnaires after a femoral nerve block, the majority of the patients felt that they could have been discharged even earlier. However, two of the patients who did not believe they were ready for early discharge due to muscle weakness.14
Singelyn et al17 compared PCA, femoral nerve block and epidural analgesia to see which method had the greatest efficacy for total hip arthroplasty. The analgesia was comparable between the femoral nerve block and the epidural groups. Both epidural and PNB groups showed significantly better analgesia than the patients receiving morphine. Although analgesia was comparable between the epidural and PNB groups, side effects were less frequent and less severe in the femoral nerve block group compared to the epidural and PCA group. This observation led investigators to the conclusion that the femoral nerve block appeared to be the best analgesia technique for total hip arthroplasty. Although, Singelyn et al17 did not study how each technique compared in relation to length of hospital stay, it is fair to assume that patients who have fewer side effects generally have shorter hospital stays. Maurer et al68 evaluated the effect of single injection blocks, continuous injection blocks, and patient-controlled analgesia for hip arthroplasty. Although the study did not gauge discharge time specifically, they noted that pain scores with continuous injection blocks were significantly lower when compared to single injection blocks and patient controlled analgesia. Maurer et al also noted a significant difference in hemodynamic stability between the groups: continuous injection blocks kept postoperative blood pressure more stable.
White et al.18 investigated effects of PNBs on the length of hospital stay. Using bupivacaine and a saline control, they compared postoperative narcotic use in patients that were allowed to supplement their pain with narcotic PCA. Results showed that patients who received intra-operative bupivacaine higher satisfaction scores, less narcotic use, lower maximal pain scores, and 40% of the patients were able to be discharged on the same day as the surgery. The study found that the average length of stay for patients with the bupivacaine PNB was 0.7 days, whereas patients without the bupivacaine spent an average of 1.4 days in the hospital.16 The authors also noted that patients who received the PNB spent less time in the PACU after surgery; however, this relationship was not found to be statistically significant.18 In a similar study, Hadzic et al69 showed that, when comparing PNBs with general anesthesia for hand surgery, patients anesthetized with PNB were able to ambulate significantly earlier (82 +/- 41 min) while the general anesthesia group ambulated slower (145 +/- 70 min). Also, the time to discharge was significantly shorter for patients in the PNB group.
A recent study conducted by Ilfeld et al70 examines pain control following total knee arthroplasty. The study shows that continuous femoral nerve block offers a viable, alternative option as compared to opioid or epidural analgesia. They noted that patients who received a continuous block instead of epidural (or patient-controlled analgesia) were able to reach their discharge criteria significantly faster (25 hours as compared to control averages of 71 hours). A similar study by Chelly et al16 found that, in total knee arthroplasties, the PNBs reduced postoperative morphine consumption and provided a reduction in recovery time compared to epidural and patient-controlled analgesia. Specifically, there was a 90% reduction in adverse events and length of stay was reduced by 20%. Two reasons emerged to explain these findings. The first was the decrease in serious complications such as postoperative bleeding, constipation, pruritus, and respiratory depression. The second was that the use of femoral block led to increased passive knee flexion during the first three postoperative days, allowing earlier mobilization of the limb.16 Capdevila et al71 performed another study on the effect of femoral nerve blocks on knee surgery. They showed that a femoral nerve block and epidural analgesia provided better postoperative knee mobilization compared to patient-controlled analgesia. As a result, the average stay in the rehabilitation center was shorter: 37 days for epidural, 40 days for femoral block and 50 days for patient-controlled analgesia.71 Although the epidural had the shortest average stay at the rehabilitation center, side effects were more frequent in epidural anesthesia (compared to femoral block).71
Salinas et al72 compared the use of single injection and continuous femoral nerve blocks on hospital stay. They found that, although the analgesia did improve with continuous femoral blocks, the length of stay was unchanged when compared to single injection femoral nerve block. The study concluded that changing a single treatment modality will not have an effect on the total hospital time because of the improved ability of physical therapy and rehabilitation. However, this study did not compare the length of stay between multiple modalities of analgesia. If the nerve block groups were compared to other modalities such as PCA or epidural techniques, there may be a significant difference in length of stay.
Hadzic et al59 performed a study that compared brachial plexus nerve block with general anesthesia for hand surgery. The results showed that 79% of the nerve block patients met criteria to bypass the Post-Anesthesia Care Unit (PACU) compared to only 25% of patients who received a general anesthetic. Similarly, none of the patients in the PNB group required pain medications during hospitalization whereas 48% of general anesthetic patients did.59 The patients who received nerve blocks were also found to ambulate earlier and were discharged sooner.
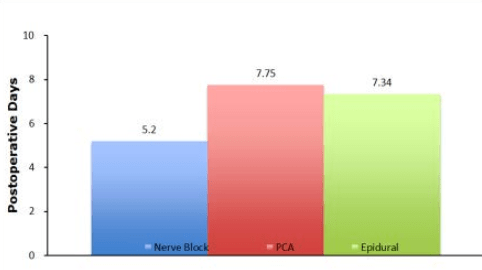
According to the literature reviewed and compilation of the data in Figure 5 it seems that PNBs are efficacious in reducing the length of stay compared to PCA. This difference could be attributable to a few variables. First, reducing the patient’s perceived pain leads to earlier discharges. Second, because it decreases opioid use, PNBs tends to have fewer opioid-related side effects. Lastly, although data is lacking in human models, PNBs reduce inflammatory mediators, allowing them to increase the speed of recovery. Additional research is needed to compare the relative length of stay in PNBs and epidurals.
Figure 5: Compilation of data from multiple studies in the literature showing the average length of stay postoperatively comparing nerve blocks, epidurals and opioid PCA. The data shows that the average length of stay when using nerve block analgesia is shorter when compared to PCA and epidural analgesia.
CONCLUSION
Adequate perioperative pain control is essential for the recovery of surgical patients. Pain associated morbidity reduces healing, increases healthcare costs and prolongs hospital length of stay. The data collected and presented in this review demonstrate the utility of PNBs in a muti-modal pain management regimen.
PNBs decrease opioid consumption resulting in less opioid related side effects. This benefit alone leads to shortened length of stay and improved patient satisfaction just by avoidance of opioid related side effects.
In regards to inflammation and surgical stress response, more research is needed. Murine studies demonstrate PNBs influence on modulating the inflammatory response to surgical trauma. Further studies are warranted to see how this research translates to humans. While epidural and spinal anesthetics have been shown to decrease the stress response, the data is limited on PNBs. Further studies are needed on the ability of PNBs to limit the surgical stress response and to determine which outcomes are affected by doing so.
With increased economic pressure for improved quality of care, lower expenditures, and shortened duration of hospitalization, a perioperative pain regimen that can control pain as well as decrease comorbidities associated with pain and or its treatment is ever so important. In many cases, including PNBs in a multimodal approach is an important part of meeting these goals.
CONFLICTS OF INTEREST: None.










