INTRODUCTION
Date palm (Phoenix dactylifera L.) is a dioecious and a wind-pollinated species. The fine powder used to pollinate date palm females is commonly known under the name of pollen. The latter is the male gametophyte used for the safe transportation of the genetic material. It is formed in the anther and germs by generating a tube that allows the ovule fertilization in female stigma. Pollen is a natural source of biochemical and nutritional substances such as protein, carbohydrates, amino acids, minerals, sterols, hormones, and many different kinds of enzymes and cofactors.1 Its composition may widely vary between species. For example, maize pollen is characterized with 23.80% protein, 13.92% oil, 22.04% total sugar,2 while hardy kiwi pollen has 26.5% protein, 7% fat and 5.3% ash.3 The physicochemical composition of pollen may be affected by other conditions such as collecting manner (bee or manually collected) and storage conditions.4 The proportions of different constituents can also vary due to the time, the season of collection and the location of the plant from which pollen was gathered.5
Pollen contains bioactive molecules such as polyphenolic compounds and flavonoids, well-known by their antioxidant capacity which contributes in the antiaging, anticarcinogen, antiinflammatory and cardioprotective effects.2,6
Throughout history and around Arab world, date palm pollen (DPP) has been used in folk medicine to enhance fertility. Investigators showed a great improvement of the reproductive system of adult male rate, rabbit, and human being after being treated with DPP.7,8,9 On the other hand, pollen has been used in food industry as a dietary supplement in yogurt, for example.10 However, the latter study was only based on the antioxidant and antimicrobial activities of honey bee collected pollen. Then, to the best of our knowledge, there are no reports related to other properties of date palm pollen that could affect the quality of food systems such as surface and thermal properties.
Tunisia is considered as one of the date-producing countries counting around 5.4 million date palm trees. In the region of Sfax from Tunisia, many male date trees are discarded since every 50 female trees require 1 male tree as a pollen source.11 Hence, the aim of this study is to add value to this discarded product. As a first step, we will focus on investigating the morphology, the physico-chemical, the surface and the antioxidant properties as well as the nutritional value of Tunisian date palm pollen. Then, we will discuss the possibility of a further cracking of this typical product to create novel ingredients acting as a bio-surfactant and replacing the synthetic antioxidants at the same time. Thus and as far as we know, this present work will be the first to relate physical to chemical properties in order to discuss the storage conditions, the way of processing and the probable behavior of DPP in agri-food and pharmaceutical field.
MATERIALS AND METHODS
Raw Material
Date Palm Pollen was manually collected from male date palm trees variety Deglet Nour aged at least 10-years, April 2015, Sfax, Tunisia. Spathes were gently shaken to separate pollen flour from flowers then sieved to remove residual particle and directly frozen in a watertight container at -20 °C for further uses.
DPP was analyzed for their properties, and all analytical determinations were performed in triplicate. Values were expressed as the mean±standard deviation.
Physico-Chemical Properties of Date Palm Pollen
Dry matter and mineral content: Dry matter (DM) and mineral content were determined according to the Association of Official Analytical Chemists.12
Proteins: Protein concentration was determined using a Dumas Elementar Rapid N cube 161 15054 (Donaustrasse, Germany).
Total protein was calculated using a nitrogen conversion factor of 6.25.13
Fats: Fats were determined using an automated Soxtherm S 306 AK (GERHARDT, Germany) equipped with six soxhlet posts and a command unit. DPP was hydrolyzed with the standard method.12
Sugars content: DPP (200 mg) were extracted using 20 mL of ethanol (80%). The mixture was magnetically stirred for 30 min then centrifuged 10 min at 10000 rpm.2 Twenty µL of filtered supernatant were analyzed with high-performance liquid chromatography (HPLC) equipped with Aminex HPX-87H column whose temperature was maintained at 40 °C. The mobile phase was sulfuric acid 3 mM in water and the flow rate was fixed at 0.4 mL/min. Glucose, fructose, and sucrose were identified and quantified using standards.
Amino acid analysis: The amino acid composition was assessed using HPLC. The sample was treated as described by Ghribi, Gafsi, Blecker, Danthine, Attia and Besbes.14 The HPLC system (biochrom) was equipped with a UV-vis detector with two wavelengths, 440 nm, and 570 nm for the proline and the other amino acids, respectively, and a cation exchange column (200×4.6 mm). Cysteine and methionine, sulfur-containing amino acids, were quantified after a pre-hydrolysis oxidation with performic acid. The contents of the different obtained amino acids were expressed as g/100 g protein.
Water activity and pH: Water activity was measured at 20 °C with a Novasina, lab swift aw-meter, Switzerland.
A 1:4 (w/v) suspension of DPP in distilled water was prepared and pH was measured with a pH meter (827 pH lab Metrohm, Switzerland).
Color: The Cie Lab parameters (L*, a*, b*) were determined using a color flex EZ (Hunterlab, USA), calibrated with black and white tile. The L* value is a measure of lightness, ranging from 0 (black) to 100 (white); the a* value ranges from -100 (green) to +100 (red) and the b* value ranges from -100 (blue) to +100 (yellow). For Hue color index, 0° or 360° represents red and 90°,180°, and 270° represents yellow, green and blue, respectively.
The Hue angle (h°) and Chroma or intensity (C*) were calculated according to the following equations:
C*=(a*²+b*²)½
h°=arc tang (b*/a*)
Particle Size
The particle size distribution was measured using a laser light diffraction instrument (Malvern Mastersizer 2000. UK). The particle size that was expressed as d (0.1) and d (0.5). d (0.5) corresponds to the mean particle size for which 50% of the sample volume is below.
X-Ray Diffraction
A D8-Advance Diffractometer (Brüker, Germany) (k Cu = 1.54178 Å, 40 kV, 30 mA) equipped with a Lynxeye detector (Brüker, Germany) was used to study the (XRD) patterns of DPP sample. The data were collected in the 2θ ranges 5-40° with a step size of 0.02° and a counting time of 0.5 s/step.14
Scanning Electron Microscopy (SEM)
DPP scanning electron microscopy images were gathered using a scanning electron microscope (JSM-5400, JEOL, Japan). Pollen flour was placed on a copper holder and coated with a fine gold layer using fine coat, JFC-1100 E, Ion sputtering device, JEOL, Japan. Then, the sample was observed at an accelerating voltage of 15 KV and at 500, 2000 and 5000-fold magnifications.
Bioactive Compounds of Date Palm Pollen
Total phenolic: 2 g of DPP were mixed with 20 mL of acetone (50%) and the mixture was shaken 2 h at 25 °C, then centrifuged 20 min at 4500 rpm. The extraction was repeated twice to enhance polyphenols extraction.15
The Folin-Ciocâlteu method was used to determine the total phenolic content. The extract (500 µL) was transferred into a tube containing 2.5 mL of solution Folin-Ciocâlteu 1:10 in water. Two mL of sodium carbonate solution 7.5% (w/v) was added. The tubes were kept at room temperature for 15 min. Then, the absorbance was measured at 765 nm against distilled water as the blank sample.16
The total polyphenol content was expressed as Galic acid equivalents (GAE) in mg/100 DPP. Galic acid was used to obtain standard curve with concentrations varying between 0-50 mg/L.
Flavonoids: The previously described extract (250 µL) was transferred into a tube containing 1 mL distilled water and 150 µL NaNO2 15 % (w/v). Six min later, 75 µL AlCl3 10 % (w/v) were added. Finally, 1 mL 1 mol/L NaOH was added and the final volume was made up to 2.5 mL of distilled water. After 15 min incubation, the absorbance was measured at 510 nm against distilled water as the blank sample. Flavonoids content was expressed as quercetin equivalents (EQ) in mg/100 g DPP.17
Nitrogen Solubility Index
DPP flour was dispersed in distilled water and pH was adjusted to value 2-12 using 1 mol/L NaOH or 1 mol/L HCl. Dispersions were shaken 30 min at 25 °C, and finally centrifuged 5000 rpm for 15 min. Protein content in the supernatant was determined using Bradford method. Bovine serum albumin (BSA) was used to obtain standard curve from 20 to 200 µg/mL. The absorbance was measured at 595 nm. Solubility was calculated as the percent of protein in supernatant to the initial protein content in the sample.
Zeta Potential
A 0.1 g/100 mL protein dispersion from pollen was prepared prior to the measurement of the surface charge using a Delsa Nano C Instrument (Malvern Instruments, Westborough, MA, USA). The isoelectric point corresponds to the pH where the surface charge is null.
Surface Tension Measurement
A 1 g/100 mL DPP aqueous dispersion was prepared for the measurement of surface tension using an automated drop volume Tensiometer TVT1 (Lauda, Germany). The measurements were in dynamic mode with a syringe volume 2.5 mL, a drop creation time from 0.07 to 0.8 s/µL and at 25±0.5 °C. The lifetime of the drops was measured as a function of their volume, which made it possible to calculate the surface tension.18
Thermal Properties of Date Palm Pollen
Differential scanning calorimetry: Differential scanning calorimetry (DSC) was performed on DPP. Heat flow was recorded during heating from -50 and 250 °C at a scan rate of 5 °C/min with Thermal Analysis Instruments Q1000 DSC (TA DSC Q1000, New Castle, DE, USA). The sample was placed in hermetic aluminum-pans and an empty one having an equal mass of 0.1 mg was used as a reference. The temperature was calibrated with two standards (Indium, Tonset: 156.6 °C, DH: 28.7 J/g) and (Eicosane, Tonset: 36.8 °C, DH: 247.4 J/g). Specific heat capacity (Cp) was calibrated using a Sapphire. The cell was purged with Nitrogen 50 mL/min. The analyzed sample mass was about 2 mg and the experiment was carried out in triplicate.
Thermogravimetric analysis: The thermogravimetric analyzer (Mettler Toledo DSC/TGA 1 star system) was used to measure weight change during heating the sample from 25 to 800 °C with a step rate of 5 °C/min. DPP (10 mg) was placed in a ceramic pan. The Nitrogen gas flow rate was kept constant at 35 mL/min. The experiments were performed in triplicate to test the repeatability of the device. Data was collected automatically to get the weight loss rate curve.
RESULTS AND DISCUSSION
Physico-Chemical Composition
The physicochemical composition of DPP, presented in Table 1, showed that the moisture content was 30.31 g/100 g, which was comparable to the value reported for Egyptian palm pollen grains (29 g/100 g) by Hassan.1 On the other hand, DPP was characterized by a neutral pH (6.31) and a high water activity equal to 0.898. These facts required either an immediate freezing or a rapid drying in order to prevent any microbial contamination. In this study, DPP has been frozen in its natural form for further analyses.
| Table 1. Physico-Chemical Properties of Date Palm Pollen |
|
Components
|
Values
|
|
Moisture (%)
|
30.31±0.86 |
| aw |
0.898±0.001
|
|
pH
|
6.31±0.01 |
| Ash % (DM) |
6.16±0.36
|
|
Protein % (DM)
|
38.18±0.60 |
| Fat % (DM) |
10.24±0.19
|
| Total sugars % (DM) |
|
Glucose % (DM)
|
3.66
|
|
Fructose % (DM)
|
4.48 |
| Sucrose % (DM) |
10.08
|
| Cie Lab parameters |
|
L*
|
84.37±0.09
|
|
a*
|
-1.11±0.02 |
| b* |
18.51±0.15
|
|
C*
|
18.55±0.15 |
| h° |
86.57±0.08
|
| Particle size (µm) |
|
d (0,1)
|
12.75±0.12 |
| d (0,5) |
28.80±0.64
|
|
Total phenols (mg AG/100 g)
|
909.30±1.12
|
| Bioactive compounds |
|
Flavonoids (mg EQ/100 g)
|
4.31±0.26
|
| DM: Dry Matter, All the data are expressed as mean±SD and are the mean of three replicates. |
The major component of DPP was proteins (38.18 g/100 g DM) (Table 1). This result was higher than that found by Hassan.1 for date palm pollen (31.11 g/100 g DM) but similar to that found by Khider et al10 for Egyptian bee-collected date palm pollen (38.06 g/100 g DM). However, it is lower than the value given by Human et al4 for the pollen of Aloe great head ii var. davyana (50.8 g/100 g DM). Thus, we may conclude that protein content can be related to the pollen source, the origin of vegetable material and climatic conditions.10 From data collected, it was clear that DPP is a potential source of proteins that can be useful in agri-food and pharmaceutical fields.
DPP presented a relatively low amount of fats (10.24 g/100 g DM) when compared with the results of Hassan,1 which showed that the fat content of DPP was 21 g/100 g DM. According to Hassan,1 this fraction contains various saturated, monounsaturated and polyunsaturated fatty acids and the predominant fatty acids of date palm pollen grain were palmitic (C16:0), linoleic (C18:2) and myristic (C14:0) acids.
DPP can be considered as a rich source of soluble sugars (18.22 g/100 g DM). Sucrose (10.08 g/100 g DM) was detected as the major sugar in the DPP sample, followed by fructose (4.48 g/100 g DM) and finally glucose (3.66 g/100 g DM). Hence, the physicochemical composition of DPP is an indicator of its high nutritive quality
Concerning color coordinates, DPP powder was characterized by a high value of lightness (L*) equal to 84.37. The coordinate b* indicating the yellow color was equal to 18.51 and the coordinate a* measuring the red color was -1.11 (Table 1). From these values, DPP was a bright yellow flour. Therefore, the addition of DPP in food will not be the origin of any undesirable color. DPP seems to be a suitable food ingredient that guarantees a favorable organoleptic quality of food system.
Particle size characteristics revealed that DPP was composed of small particles. In fact, 10% and 50% of the particle do not exceed 28.80 µm and 12.75 µm, respectively. This size promotes functional properties such as water and oil holding capacities since small particles allow more contact with fluids.19
Amino Acid Analysis
The amino acid composition of the current studied DPP was presented in Table 2. The essential amino acids (Valine, Histidine, Isoleucine, leucine, lysine, methionine, phenylalanine, threonine and tryptophane) presented around 39% of the total detected amino acids in the analyzed sample, which indicated the great nutritional value of DPP. However, our value was lower than that reported for Egyptian date palm pollen (47%).1 This slight dif ference was due to the variations observed in histidine, leucine, lysine and methionine content. Compared with other type of pollen, it was clear that DPP exhibited a relatively high amount of essential amino acids, followed by fresh Aloe great head ii var. davyana pollen (33%),4 hardy kiwi pollen (30%) and finally oak pollen (23%).3On the other hand, the observed percentages remained important for human nutrition since they exceeded those reported by WHO/FAO/UNU (2007) (Table 1).
| Table 2. Amino-Acid Composition of Date Palm Pollen |
|
Amino acids (g/100 g protein)
|
DPP |
WHO/FAO/UNU (2007) |
| Valine |
5.16 |
3.9
|
|
Histidine
|
2.53 |
1.5 |
| Isoleucine |
4.37 |
3
|
|
Leucine
|
8.35 |
5.9 |
| Lysine |
7.73 |
4.5
|
|
Methionine
|
2.37 |
– |
| Phenylalanine |
4.25 |
3.8
|
|
Threonine
|
4.70 |
2.3 |
| Total essential amino acids |
39.46 |
–
|
|
Tyrosine
|
3.46 |
– |
| Arginine |
5.77 |
–
|
|
Alanine
|
6.48 |
– |
| Aspartic Acid |
10.41 |
–
|
|
Glutamic Acid
|
13.23 |
– |
| Glycine |
5.00 |
–
|
|
Cysteine
|
1.11 |
– |
| Serine |
5.74 |
–
|
|
Total Non essential amino acids
|
51.20 |
– |
| Total sulfur amino acids |
3.48 |
–
|
| E/T (%) |
43.53 |
– |
As can be observed from the obtained results, among seventeen defined amino acids, glutamic acid (13.23%) was the major one followed by aspartic acid (10.41%), leucine (8.35%), lysine (7.73%) and alanine (6.48%). Glutamic and aspartic acids are responsible for a palatable taste, they are also considered as medicinal amino acid since aspartic acid has an anti-fatigue, antitussive and expectorant effect and glutamic acid is the most abundant free amino acid in skeletal muscle, it plays an important role in the synthesis of purines and pyrimidines by donating nitrogen.20 The second group of amino acids was present in less percentage. Examples of these amino acids are arginine, valine, glycine, serine, threonine, phenylalanine and isoleucine, whose contents ranged between 3.46% and 5.77%. Alanine and glycine have a sweet taste. Besides, glycine is one of the major components of human skin collagen. It interferes with other essential amino acids to form a polypeptide that would promote regrowth and tissue healing, while arginine stimulates the secretion of pituitary and pancreas gland hormones, enhancing immunity and reducing the tumor protein synthesis and growth.20 Moderate amounts of other amino acids were detected, such as histidine (2.53%), methionine (2.37%) and cysteine (1.11%). It was worthy to note that cysteine and methionine were the two least noted amino acids in other types of pollen.3,4 Then, we might deduce that the amino acid composition depends on pollen’s origin as well as the climatic and nutritional conditions of the plants on which the pollen matures.1 Therefore, DPP could be used as a raw material to improve food’s flavor and nutritional quality.
Bioactive Compounds
The obtained results (Table 1) showed that the phenol content of DPP is 909.30 mg EAG/100 g DM, which was in accordance with the data provided by Žilić et al2 since maize pollen presented an average of 889.02 mg EAG/100 g. This value proved that DPP can be considered as a rich source of natural bioactive compounds that can be used to prevent the oxidation of many food products. Such data could be of great interest for consumers, since phenolic substances act as an antioxidant, and present multiple biological effects, including the reduction of the risk of heart disease, cancer, cataracts.21 These compounds also prevent the oxidation of LDL lipoprotein, platelet aggregation and damage to red blood cells.22
Additionally, DPP had a very low content of flavonoids 4.31 mg EQ/ 100 g compared to date palm pollen 266 mg EQ/ 100 g and Mimosa pollen 548 EQ/100 g.23 This difference could be explained by the fact that the total phenolic and flavonoids contents could be influenced by geographical origin, storage and drying procedure.24 In addition, solvents used for the extraction process and its polarity could play a major role in increasing phenolic compounds recovery.15
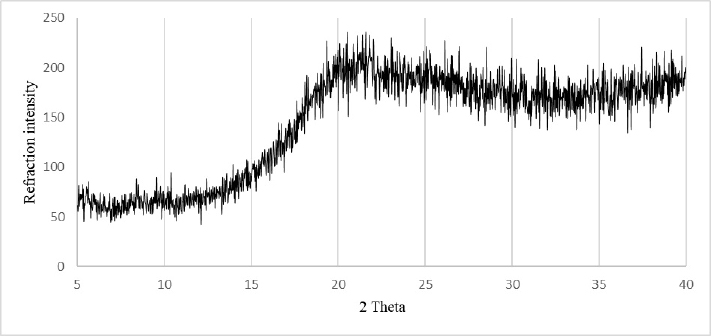
X-Ray Diffraction
X-Ray diffraction (XRD) was performed to study DPP structure. Figure 1 revealed that XRD patterns did not show any band over the 2θ ranges 5-40°. The DPP had a dominant amorphous halo. Such amorphous structure may cause stability problems, while preserving DPP powder. However, it leads to better techno-functional properties when compared with crystalized flours. Thus, it is important to store DPP in a water-air tight container to preserve its quality.14
Figure 1. X-Ray Diffraction Pattern of Date Palm Pollen
Pollen Morphology by Scanning Electron Microscopy
Pollen morphology is an expression of its genome whose variability may be utilized in species identification.25 Thus, DPP was submitted to scanning electron microscopy in order to investigate pollen’s structure. The obtained SEM images proved that the morphology of the currently studied pollen was similar to that presented by Bishr and Desoukey.5 In fact, the SEM analysis of DPP (Figure 2) showed monad, elliptical and bilateral particles with small size ranging between 10 and 25 µm. The DPP grains were characterized by one aperture from which the pollen tube grows, and many pores of various diameter ornamenting the exine surface, allowing the exchange of different substances with the outer environment. Exine’s characteristics have been used in some studies to allow molecules to penetrate in the pollen grain. In fact, Atwe et al26 demonstrated that the pollen grains of Lycopodium Clavatum can be evacuated through exine, which provides a clean spore filled with vaccine molecules for oral vaccination.
Figure 2. Scanning Electron Microscopy Images of Date Palm Pollen

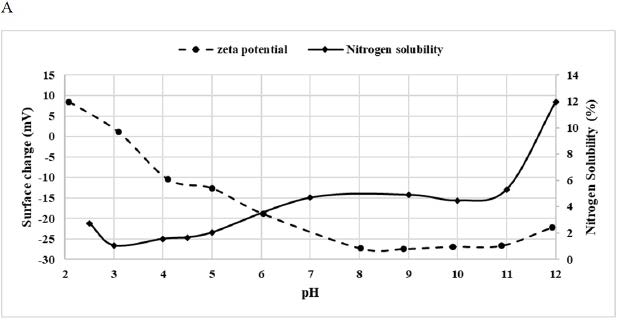
Nitrogen Solubility Index
DPP solubility, as well as its surface charge, were presented in Figure 3A. Nitrogen solubility depends on suspension’s pH, it increased when pH increased. Indeed, it was minimal (1%) at pH 3 and maximal (12%) at pH 12 (Figure 3A). These values were very low when compared with other flour’s solubility. For example, Wani et al27 studied the protein solubility of Indian kidney bean flour and found that the minimum of solubility (7.1%) occurred at pH 4 near the isoelectric point and the maximum was at pH 10 with an average of 96.55%. This low solubility value could be attributed to the structure of pollen grain which is composed of two layers, an outer exine, and an inner intine. The exine is composed of a strong biopolymer called sporopollenin which is resistant to acetolysis and high temperature while the intine is mostly made of cellulose and pectin.26
Zeta Potential
Zeta potential analysis (Figure 3A) showed that the surface charge of DPP flour also depends on pH. Indeed, the more the pH was alkaline the more the surface charge was negative. This fact led to less attraction between proteins and increased solubility. The surface charge was the least negative at pH 3, which corresponded to the isoelectric point where the least solubility value was noticed.28 The solubility profile could be exploited in further protein extraction.
Figure 3. Surface Properties of Date Palm Pollen
A: Effect of pH on the Zeta Potential and Nitrogen Solubility
B: Surface Tension (1% Aqueous Suspension)
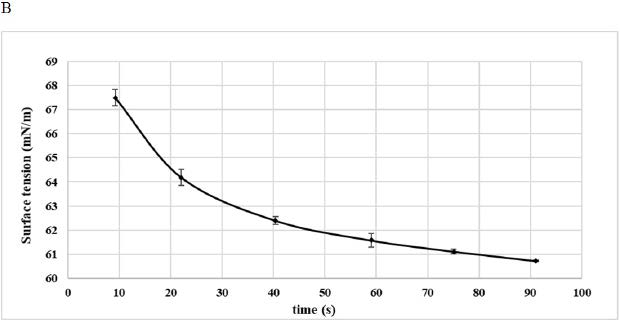
Surface Tension Analysis
The ability of 1 g/100 mL DPP flour aqueous suspension to reduce the interfacial tension was studied in Figure 3B. As can be observed from the figure, the curve could be divided into two phases, a first one characterized by a sharp decrease in the surface tension from 67.5 mN/m to 61.5 mN/m and a second phase leading to the equilibrium of the surface tension near 60 mN/m (Figure 3B). These values were important when compared with values reported for date seed fibro protein extract Deglet Nour (1 g/100 mL protein concentration) that reduced the surface tension to 57.4 mN/m.18 From all results, it was obvious that DPP could act as a surfactant thanks to its hydrophobic-hydrophilic proportion mainly existing in the protein fraction. In fact, this compound could reach the interface, leading to the reduction of interfacial tension. Lowering the surface tension may be the origin of a great foaming and emulsifying properties, which could improve food quality.29
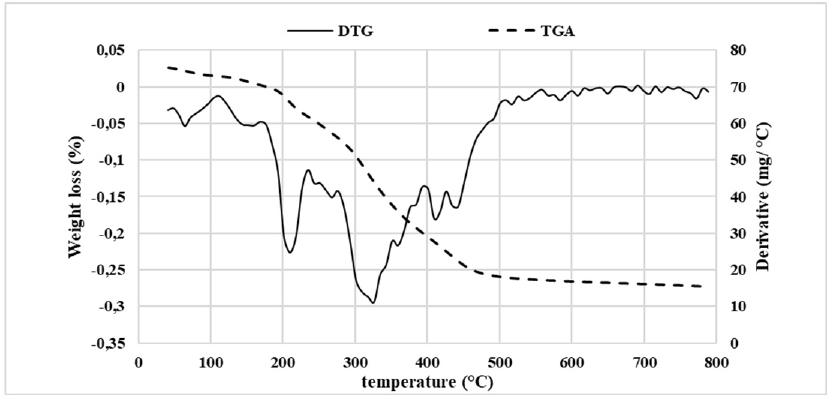
Thermal Properties
Date palm pollen was submitted to the thermogravimetric technique in order to investigate the thermal behavior of the flour. Figure 4A showed six events characterized by different weight losses. While the first step occurred up to 100 °C and represented a weight loss of approximately 27%, the second event with a weight loss of 2.57% took place between 100 °C and 170 °C. Both steps were mainly attributed to the water loss. Indeed, the moisture determined with gravimetric method corresponds to 31%, which was the sum of the weight loss of both steps.
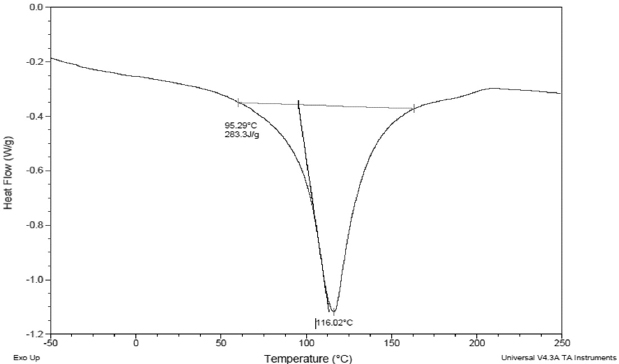
Figure 4. Thermal Properties of Date Palm Pollen
A: Thermogravimetric Curve
B: Differential Scanning Calorimetry Curve
The third and fourth steps with a weight loss of 8.4% and 5.55%, respectively, were between 170 °C and 275 °C. This weight loss was due to successive thermal degradation reaction of carbohydrates and organic compounds, such as triterpenes, alkaloids, and flavonoids.30
In the fourth step, 27% of the total weight was lost between 275 °C and 400 °C. This was the result of a complete decomposition of carbohydrates, the beginning of the carbon formation and the elimination of volatile materials. The final event representing a weight loss of 11.7% refers to the oxidation of carbonaceous materials and the formation of ash.30 From the collected data, we might conclude that the thermal decomposition of DPP flour occurred in a progressive way owing to the difference in the stability of different components against thermal treatment.
Differential scanning calorimetry (DSC) is an important tool that allows studying thermodynamics transitions. DSC Thermogram (Figure 4B) showed only one endothermic peak at 116.02 °C related to the transition of the protein from native to denatured form. This value was higher than the denaturation temperature of desi chickpea protein isolates which ranged between 98.5 and 99.8 °C.31 However, oven and freeze-dried chickpea protein concentrates were more stable since the temperature of denaturation had an average of 131 °C, which was explained by the fact that proteins had lost their native form during drying.14 DPP flour was conserved at a very low temperature (-20 °C) which preserved protein’s quality. As a conclusion, DPP proteins seemed to be thermally stable during storage and in the different food systems.
CONCLUSION
The obtained data clearly proved the substantial nutritional value of date palm pollen due to the exceptional content of protein, amino acids, phenols, and flavonoids. The amorphous structure determined using X-ray diffraction proved that DPP could present great techno-functional properties while stored in suitable conditions. Lowering the surface tension using DPP yields interesting results for using it as a novel efficient natural surfactant as a whole or by the extraction of protein fraction to improve the surfactant properties. Therefore, date palm pollen is recommended to be used as an ingredient in functional food to ameliorate its nutritional and organoleptic quality.
ACKNOWLEDGMENT
This work was funded by the Ministry of Higher Education and Scientific Research–Tunisia and Wallonie Bruxelles International (project 9, axis 2)
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.











