INTRODUCTION
Over 95% of cassava produced in Nigeria is used for human food. However, a cassava rootbased diet does not provide complete nutrition because it contains 85% starch and only 1-2% protein. Additionally, toxic compounds and rapid deterioration after harvest limit the utilization of unprocessed cassava.1
Fufu is a fermented wet cassava paste that is widely consumed in eastern and southwestern Nigeria and other parts of West Africa. However, fufu consumption has decreased due to inherent odor, short shelf life and tedious preparation.2 Regular fufu is made by fermenting freshly harvested cassava tubers in water in open containers for days and is traditionally sold in the highly perishable wet form.1 The short shelf life is a serious limitation for large-scale processors. A practical approach for improving the shelf life and marketability of fufu is drying, which is aimed at producing reconstitutable fufu dough with physico-chemical characteristics of cooked wet paste.2,3 While several methods have been used for the drying of fufu, it was determined that a rotary dryer provided a more acceptable product compared to cabinet and sun drying methods, even though the rotary drying method was not cost effective.4 In addition, as reported by Sanni et al2 several drying techniques have been reported to reduce the strong odor of fufu, but the products were sticky, bland and the quality unacceptable when reconstituted from flour.
The objectives of this study were to determine the characteristics of protein- and pro-vitamin A-fortified fermented cassava flours with or without the addition of Lactobacillus plantarum strain 6710 and their influence on the processing of cooked fufu. Similar characteristics for cooked fufu from protein- and pro-vitamin A-fortified cassava and the wild type cassava flour may allow consumers to enrich their diets nutritionally without sacrificing the inherent characteristics of commonly consumed cooked fufu.
MATERIALS AND METHODS
Cassava Flours
Four types of fortified cassava flours (zeolin (Z), sporazein (S), sporazein plus pro-vitamin A (SPRO) and pro-vitamin A (PRO)) with protein contents (wb) of 9.52, 6.83, 3.63 and 2.14%, respectively, as well as wild type cassava flour (1.41% protein), were provided by the International Laboratory for Tropical Agricultural Biotechnology (ILTAB) (St. Louis, MO, USA).
Cultures: Propagation and Storage
L. plantarum BFE 6710 strain grown as a stab culture was provided by the Max Rubner-Institut (Karlsruhe, Germany),5 and was routinely grown in Lactobacilli de Man, Rogosa and Sharpe (MRS) broth (Difco™, Sparks, MD, USA) at 32 ºC for 24 h underaerobic conditions.
After growth, culture was placed in a cryo tube with a final concentration of 20% glycerol and stored at -72 ºC for further use (stock culture). Working cultures, obtained from stock culture, were streaked on MRS agar, incubated at 32 ºC for 48 h, and colonies transferred to MRS broth. Cultures were propagated twice before use. The pre-culture was centrifuged at 8000 xg at 4 ºC for 10 min. The pellet was washed twice with buffered peptone water (BPW) (BBL™, Sparks, MD, USA), centrifuged and resuspended into 9 mL of BPW resulting in 7×1010 CFU/mL.
Growth of Starter Culture in Cassava Flour
All cassava flours were stored in sealed plastic containers at 4 ºC. Ninety grams of flour were transferred to sterilized plastic containers and inoculated with L. plantarum by transferring a cell pellet suspension (7×1010 CFU/mL). The total moisture content of the cassava sample was adjusted to 68%. A second set of non-inoculated flours was prepared. Non-inoculated (NF) and inoculated flours (LF) were covered and kept at 32 ºC for 96 h.
Fufu Preparation
Fufu flour was prepared as shown in Figure 1. Samples were removed at 0, 24, 48, 72, and 96 h of incubation, and dried for 6.5 h using a tray drier model UOP 8 (Armfield Limited, England, UK) at 70 ºC with air flow of 1.45 m/sec. Dried samples were milled using a Tecator Cemotec 1090 sample mill (FossTecator, Eden Prairie, MN, USA), set to 1, and a portion of the resulting fufu flour was set aside uncooked and stored in plastic scintillation vials at -70 ºC. Milled, fermented cassava samples with and without Lactobacillus plantarum strain at all fermentation times were mixed with water in a ratio of 1:3 (cassava: water). Mixes were cooked in a microwave oven Emerson 600 W (Model MW8665W) at the highest power setting 10(100%) in five cycles of 15 s, each followed by a manual mixing of 1 min. Cooked fufu samples were cooled at 20 ºC for 2 h before sensory evaluation. A portion of the cooked fufu samples was stored at -70 ºC until analysis.
Figure 1: Flow chart for processing of fermented cassava flour.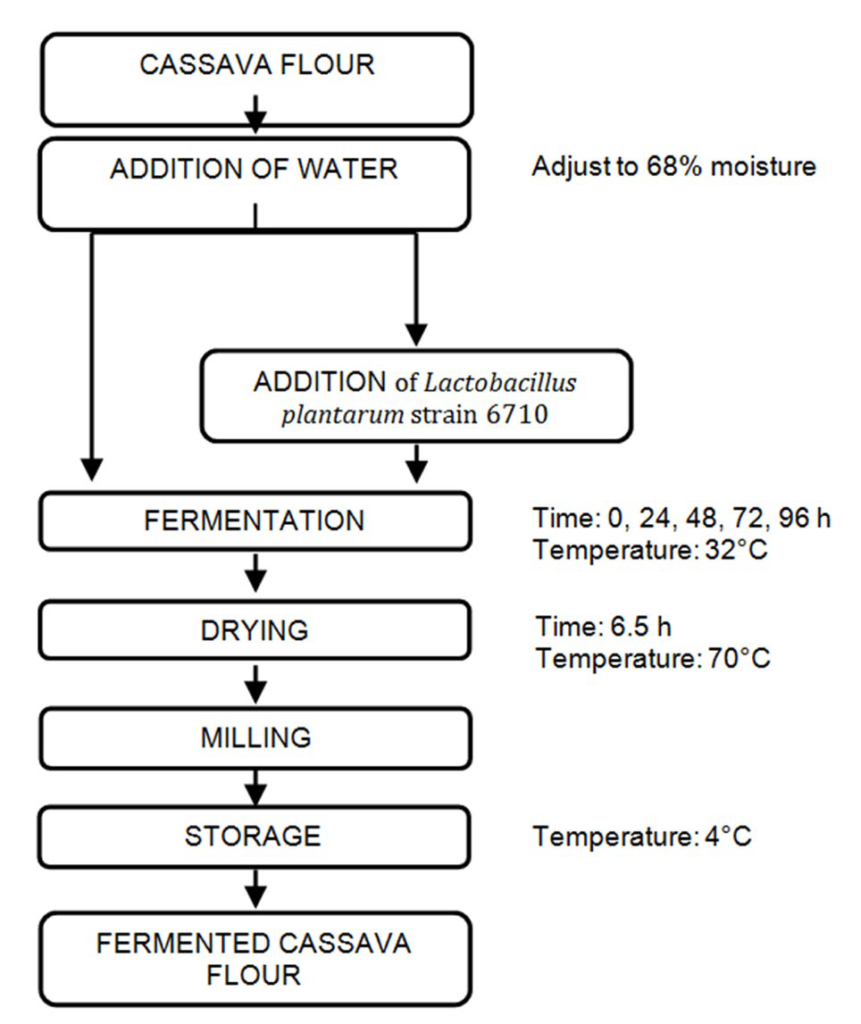
PHYSICO-CHEMICAL ANALYSIS
Pasting Properties
Starch pasting properties were measured for fufu flour with and without added Lactobacillus plantarum culture at all fermentation times. The measurements were done with a Rapid Visco Analyzer (RVA) (Newport Scientific Pty Ltd., Warriewood, Australia) interfaced with a computer equipped with Thermocline software for Windows (release 2.1) according to American Association for Clinical Chemistry (AACC) Approved Method 76- 21.01.6 Heating and cooling cycles were as follows: adjustment to 50 ºC followed by a 1 min hold, heating from 50-95 ºC over 4.42 min, holding at 95 ºC for 2.7 min, cooling to 50 ºC over 3.82 min, and a final hold at 50 ºC for 1.06 min. Peak viscosity, setback viscosity, final viscosity, pasting temperature and time to reach peak viscosity were estimated.7,8
Ph and Titratable Acidity (Ta)
Modified methods 943.02, sec. 32.1.20 and 942.15, sec. 37.1.37B were used for determination of pH and titratable acidity, respectively.9
Volatile Analysis
The method of Iyer et al10 with optimization was used for the identification of volatile compounds in cooked fufu samples. Samples (0.5 g) were mixed with sodium chloride (0.33 g) and distilled water (1.0 mL) in 10 mL headspace amber rounded bottom vials sealed with a screw cap possessing a PTFE/silicone septum. The procedure was automated using a CTC CombiPal autosampler (Zwingen, Switzerland), using Cycle Composer software version A.01.04 (Agilent Technologies Inc., CA, USA). Sample was stirred at 250 rpm and a SPME stableflex fiber coated with 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) was exposed to the slurry headspace.
The volatiles were collected for 60 min and thermally desorbed into the injection port of an Agilent Technologies 6890 gas chromatograph with a 6890 N GC split/splitless injector with data collection by Chemstation software version E.02.00.493 and a HP-5MS column (5% Phenyl Methyl Siloxane) (30 m × 0.248 mm × 0.25 µm film thickness) (J&W Scientific, Folsom, CA, USA). Helium was used as the carrier gas. The injector and detector temperatures were 200 ºC and 250 ºC, respectively. The column temperature was initially at 33 ºC for 5 min before increasing to 50 ºC at a rate of 2 ºC/min, and then to 225 ºC at a rate of 5 ºC/min. The sample was desorbed for 5 min with injector in the splitless mode. Cooked fufu volatiles were identified using a mass spectrometer MS 5975C (inert XL MSD) and MS spectra were compared against a NIST library. Data were quantitated by running standards and developing response factors based on water matrices. The final values were reported as µg/mL.
Color Evaluation
Cooked fufu (~5 g) was placed in a plastic petri dish and the color read on a Minolta colorimeter CM-2002. Values of L*, a*, and b* were recorded.11 Average values were obtained from two randomly selected points on the surface of the samples.
Instrumental Analysis of Texture
Cooked fufu hardness, adhesiveness and springiness were measured using a TA-XT2 food texture analyzer (Texture Technologies, Surrey, UK) and data were collected with Texture Expert Exceed (Version 2.64). The sample was placed between flat cross heads. A 50 mm diameter flat aluminum cylinder probe along with a flat plate on the HDP/90 heavy duty platform were used for compression of the formed sample spheres. The samples were compressed at cross head speed of 1 mm/s to 50% strain following a trigger force of 0.1 N with a 0.10 s pause between the first and second compressions. Prior to the textural measurements, fufu sample was cooked, set at room temperature for 15 min, weighed (5 g), manually shaped as a sphere and kept at room temperature for 2 hours.
SENSORY ANALYSIS
Sensory Panel Training
Five volunteers (1 male and 4 female), between 18 and 35 years old were recruited from Washington State University based on their availability and consumption of fermented cassava products at least once a week. Panelists were informed that were assessing the sensory properties of fufu. The Washington State University Institutional Review Board (IRB) for human subject participation approved this project.
Panelists were trained for 7.5 h, during which they learned definitions of the attributes, descriptors, and the evaluation technique (Table 1). A 15 cm line scale was used for each descriptor, anchored by the terms ‘low’ (1 cm) and ‘high’ (14 cm). Commercial fufu flour (Kum-Koum) from Cameroon (JKUB, LLC International Foods, and Washington, DC, USA) was used for training
Table 1: Attributes, definitions, evaluation technique, standards and intensity values (I) used for sensory evaluation of proposed fufu cassava product.
|
ATTRIBUTE/ Descriptors
|
DEFINITION |
TECHNIQUE |
STANDARDS
|
| VISUAL |
| Color intensity for brownness |
The extent to which the color of the sample is intense. The low end of the scale represents a dull brown color and the high end of the scale represents the brown color |
Compare to color chips for brownness |
Color chips Source: ACE paint colors for your life (ACE N° 9960485). Codes: C20-1 C20-4 C20-2 C20-5 C20-3 C20-6 |
| AROMA |
| Fermented aroma |
Intensity of fermented cassava aroma |
*Swirl the bottle containing the standard. *Remove lid. *2-3 short, sharp sniffs. *Replace lid. |
Shredded cassava solution: 1:3 (cassava:water) with 550 µL acetic acid (I:12) |
| Stale aroma |
Intensity of stale aroma |
*Swirl the bottle containing the standard. *Remove lid. *2-3 short, sharp sniffs. *Replace lid. |
Corked standard solution (Wine awakening): 1 drop of standard + 200 mL water (I:12.5) |
| Fufu aroma |
Intensity of fufu aroma |
*Swirl the bottle containing the standard. *Remove lid. *2-3 short, sharp sniffs. *Replace lid. |
Fufu flour (Cameroon) solution: 1:3.75 ( fufu flour:water) (I:12) |
| TEXTURE |
| Hardness |
Force required to fully compress a sample with the fingers |
Compress the sample between the thumb and index finger. |
Frankfurter (I:13.5) |
| Adhesiveness |
Degree to which a sample adheres to the fingers |
Press the sample between the thumb and the fore-and middle fingers, then release; evaluate the degree of adherence. |
Cooked fufu flour (Cameroon) solution: 1:3 (flour:water) (I:14) |
| Springiness |
Degree to which sample returns to original shape after a certain time period |
Press the sample by the tip of the forefinger and feel the recovery while releasing the finger. |
Bread (I:14.5) |
Formal Sensory Evaluations
Cooked fufu cassava products (5 g) were randomly presented to each panelist, one sample at a time, in a covered 2 oz. soufflé cup identified with a three-digit code. Panelists rated the perception of intensity of selected attributes presented in Table 1 over a period of 7 days. Filtered deionized water was provided for sniffing between samples. Within each session, 15 flights were presented. A period of 10 min was imposed after the seventh flight to rest the senses. Each cooked fufu product was evaluated in a randomized complete block design. All sessions were carried out in separate booths under white light equipped with a computerized system and sensory software (Compusense® five software (5.2, Guelph, ON, Canada) for data collection.
DATA ANALYSIS
Physico-chemical data of dried fermented cassava flour (fufu flour) and/or cooked fufu samples were analyzed for significant differences using a three-way analysis of variance (ANOVA). Tukey’s multiple comparisons of means were used to analyze both dried fermented cassava flour (fufu flour) and cooked fufu physico-chemical data with XLSTAT (Version 7.5.3, XLSTAT Addinsoft, France, Paris) at the p≤0.05 confidence level. The ANOVA performed on physico-chemical data used sample, fermentation, and time as fixed effects. Sensory data of cooked fufu samples were analyzed for significant differences using a threeway analysis of variance (ANOVA) in a randomized complete block using PROC MIXED in SAS 9.1 software (SAS Inst., Cary, NC, USA). The analysis of sensory data using a mixed effects model assumed panelists as a random effect factor and sample, fermentation and time as fixed effect factors. Significance level was defined as p≤0.05. All tests were duplicated.
RESULTS AND DISCUSSION
pH and Titratable Acidity
Most naturally fermented samples (NF) reached a minimum pH value at 96 h fermentation compared to 72 h for the L. plantarum samples (LF). NFS and NF SPRO showed a pH value of 4.34 and 4.51 at 96 and 72 h, respectively. A fast decrease to pH 3.60 was observed when the lactic acid bacteria strain was added for LFS and LF SPRO at 96 and 72 h of fermentation, respectively. These results agree with Brauman et al,12 who reported that LAB produced a rapid drop in pH to ~4.5 after two days fermentation of cassava roots.
The fermentation process resulted in a gradual increase of acidity for the non-inoculated wild type (NFWT) and inoculated wild type (LFWT) cooked fufu up to 72 h of fermentation, with no further significant change at 96 h (p>0.05). An analogous tendency was shown for the NFZ and LFZ.
When Lactobacillus plantarum starter culture was added, the decrease in pH and increase in TA occurred more rapidly (24 h) when compared to samples without starter culture. This agrees with Kostinek et al,13 who determined a high capability for the obligate homo- and facultative hetero fermentative group (mostly L. plantarum) to lower the pH of the medium. Acid pro duction and subsequent decrease extends lag phase of sensitive organisms including foodborne pathogens.14
Pasting Properties
Peak time is a property that indicates the minimum time required for fufu cooking. At 0 h fermentation, significant differences in peak time, that may have developed during the pasting analysis of the samples, were found between all NF and LF individual samples of a given cassava material, i.e. NFWT versus LFWT (p≤0.05) (Table 2), with the exception of NF PRO and LF PRO. At 96 h fermentation, NF and LF treatments were significantly different in peak time for all cassava material pairs (p≤0.05) (Table 2). Peak times were consistently higher for the NF versus LF samples (within a given sample pair). This supported the observation that the decrease of pH or increase of acidity occurs more rapidly when Lactobacillus plantarum starter culture was added. Therefore, it is likely that fermentation in NF samples was focused most prevalently on amorphous regions of starch granules, which caused an increase of peak time and did not allow a more rapid fermentation. The greatest peak time was found for NF SPRO (4.87 min) at 0 h fermentation, which could be related to protein-carotenoid interaction that increased the time to peak viscosity. Such ingredients may increase the peak time if they coat the granule, limiting water penetration and thus, hydration and swelling. Protein-carotene interaction was shown by Marx et al15 when crystalline β-carotene with bovine serum albumin in a model system proved to be stable towards isomerization irrespective of time-temperature parameters which indicated binding as the protein protected the carotenoid.
Table 2: Pasting properties1 of dried fermented cassava flour “fufu flour” with (LF) and without (NF) the addition of a starter culture at 0 and 96 h of fermentation.
1Results are reported as the mean of two determinations±standard deviation. Means within the same column containing different letters are significantly different (p≤ 0.05).
2Wild type (WT), zeolin (Z), sporazein (S), sporazein plus pro-vitamin A (SPRO) and pro-vitamin A (PRO) fortified material.
|
Fermentation time (h)1
|
Cassava material2 |
Peak viscosity (cP) |
Trough (cP) |
Breakdown viscosity (cP) |
Final viscosity (cP)
|
Setback viscosity (cP)
|
Peak time (min) |
Pasting temperature (°C)
|
| 0 |
LF |
WT |
4972±8c |
1792±10d |
3189±14c |
2735±15f |
963±54c |
3.85±0.03f |
64.58±0.67de |
| Z |
5518±42b |
1628±37e |
3899±18a |
2890±8e |
1334±73ab |
3.68±0.02g |
62.13±0.04 |
| S |
4285±6f |
1672±22e |
2620±6d |
2588±14g |
959±23cd |
4.36±0.04cd |
71.08±0.11a |
| SPRO |
3667±32g |
1566±13e |
2094±8g |
2878±16e |
1325±21ab |
4.46±0.00c |
69.78±0.11bc |
| PRO |
4418±58e |
1812±11d |
2563±8e |
3249±15c |
1412±9a |
4.26±0.01d |
69.05±0.07c |
| NF |
WT |
5476±37b |
2790±13a |
2652±25d |
3949±47a |
1194±12b |
4.30±0.05d |
69.73±0.04bc |
| Z |
5969±26a |
2359±62b |
3648±18b |
3057±4d |
773±40d |
4.02±0.04e |
61.53±0.04e |
| S |
4561±40d |
1968±28c |
2649±11d |
2813±17ef |
884±44c |
4.63±0.04b |
65.35±0.07d |
| SPRO |
3328±2h |
1623±8e |
1705±6h |
2565±25g |
955 ±14c |
4.87±0.01a |
70.35±0.07ab |
| PRO |
4269±25f |
963±25c |
2315±14f |
3456±11b |
1457±35a |
4.25±0.02d |
69.08±0.11c |
| 96 |
LF |
WT |
4965±18b |
2243±22a |
2740±21c |
3244±24bc |
1005±6c |
4.14±0.01d |
60.83±0.04b |
| Z |
5179±71a |
1731±30bc |
3453±48a |
2707±16e |
996±13c |
3.77±0.05e |
71.03±0.04a |
| S |
3480±11f |
1280±13ef |
2189±8e |
2240±20g |
924±18cd |
4.36±0.04c |
72.95±0.07ab |
| SPRO |
2809±81g |
1357±59e |
1452±21f |
2045±13h |
775±50e |
4.86±0.01b |
70.98±0.04ab |
| PRO |
3633±11e |
1045±17g |
2590±10d |
3240±9bc |
2172±24a |
4.85±0.02b |
70.18±0.25ab |
| NF |
WT |
4571±12c |
1779±14b |
2818±11c |
3303±18ab |
1534±18b |
5.06±0.01a |
62.03±0.04b |
| Z |
4683±9c |
1555±43d |
3102±15b |
3066±33d |
1463±7b |
4.03±0.04d |
71.60±0.00ab |
| S |
2830±21g |
1633±14cd |
1185±18g |
3181±7c |
1551±11b |
5.08±0.02a |
75.45±0.07ab |
| SPRO |
2682±40g |
1681±21bc |
1051±10h |
2489±3f |
852±37de |
5.14±0.01a |
74.23±0.04ab |
| PRO |
3985±18d |
1239±14f |
2766±33c |
3339±42a |
2150±13a |
5.05±0.07a |
71.03±0.04ab |
In addition, NF SPRO showed the lowest breakdown values (Table 2). A similar trend was observed for LF SPRO when compared with all inoculated cassava materials at 0 and 96 h fermentation. Therefore, these fufu flours might be able to withstand heating and shear stress compared to the other samples. At 96 h fermentation, NF SPRO also showed the highest peak time (5.14 min) even though it was not significantly different from NFWT, NFS and NF SPRO. Based on the lowest peak time, NFZ and LFZ at 0 and 96 h of fermentation will cook faster with less energy consumed, thereby saving cost and time compared to other samples. Except for NFZ, peak times for NF samples at 96 h fermentation were slightly higher than the values determined for LF samples. This may be due to the conversion of starch to simple sugars due to fermentation by the microorganisms causing a decrease of the stability of the starch materials.16
Prior to fermentation, the mean peak viscosity was greatest for NFZ fufu flour (5969 cP) as opposed to NF SPRO (3328 cP). A similar relationship was observed for LFZ and LF SPRO cassava materials (Table 2). This observation might have been influenced by greater or more rapid swelling of starch granules in NFZ, which in turn may cause instability and consequently disruption upon the heating and stirring (i.e., breakdown). Furthermore, the final viscosity and pasting temperature of NFZ flour were substantially lower, likely due to free leaching of amylose and amylopectin from the granules.17 Thus, it would appear that the Z cassava materials swelled earliest and most rapidly, leading to a higher degree of breakdown.
The setback region is the phase of the RVA analysis where starch molecules start to reassociate during cooling, reflecting retrogradation of the starch molecules. Setback has been consequently correlated with the texture of the reconstituted fufu flours.3,18 Thus, a low setback value indicates low rate of starch retrogradation and syneresis. Here, at 0 h of fermentation the least extent retrogradation was shown with the NFZ fufu flour (Table 2). At 96 h of fermentation, NF PRO and LF PRO fufu flours showed the greatest setback viscosity values possibly due to increased hydrogen bonding during cooling (Table 2). This hydrogen bonding leads to the growth of gel micellar regions, hence increase in index of retrogradation, making entrapped water more prone to expression. This retrogradation as explained by Whistler and BeMiller18 also depends on other variables that include amylose/amylopectin ratio and their structures that will be based on the botanical source, temperature and starch concentration and other ingredients.
Regarding the final viscosity that indicates the ability of the material to form a viscous paste, NFWT fufu flour showed the highest value at 0 h fermentation while NFWT and NF PRO fufu flours obtained the highest final viscosity values at 96 h (Table 2).
Volatile Compounds
Only a few aromatic compounds were detected in the cooked fufu samples. A similar profile was obtained for all samples in spite of their specific characteristics. The main compounds detected were acetic acid, hexanal, nonanal and decanal at all fermentation times. At 0 h of fermentation, a low concentration of acetic acid was found in all samples (Table 3) and no significant differences were found among treatments (p>0.05). Acetic acid concentrations were greater than the published threshold value (24.3 µg/mL)19 in all cooked fufu samples fermented from 24-96 h. High concentrations of acetic acid may affect the acceptability of the cooked fufu product. Hexanal was detected in most samples at all fermentation times. No significant differences in hexanal concentration were found among samples at 48 h fermentation (data not shown). In most samples, the level of hexanal was below the published threshold value (0.03 µg/ mL).19 The greatest amount of hexanal was found in NF PRO at 24 h fermentation (0.146 µg/mL) (Table 3).
Table 3: Volatile compounds1 detected in cooked fufu with (LF) and without (NF) the addition of a starter culture at 0 h and 24 h of fermentation.
1Results are reported as the mean of two determinations. Means containing different letters within the same column are significantly different (p≤ 0.05).
2Wild type (WT), zeolin (Z), sporazein (S), sporazein plus pro-vitamin A (SPRO) and pro-vitamin A (PRO) fortified material. nd: No detectable
| Cassava material2 |
Volatile compound concentration (µg/mL)1
|
|
0h
|
24h
|
|
Acetic acid
|
Hexanal |
Nonanal |
Decanal |
Acetic acid |
Hexanal
|
Nonanal
|
Decanal
|
| LF |
WT |
21.96 |
nd |
0.007 |
0.003b |
91.99b |
nd |
0.004 |
0.002 |
| Z |
38.86 |
0.033c |
0.010 |
0.002b |
187.43b |
0.006d |
0.006 |
0.002 |
|
S
|
34.05 |
nd |
0.005 |
0.002b |
92.63b |
nd |
0.005 |
0.002 |
| SPRO |
41.58 |
0.045c |
0.008 |
0.002b |
133.06b |
0.026cd |
0.007 |
0.003
|
|
PRO
|
20.38 |
0.086a |
0.014 |
0.002b |
196.55b |
0.083abc |
0.012 |
0.003 |
| NF |
WT |
30.67 |
0.017d |
0.011 |
nd |
117.96b |
0.039bcd |
0.012 |
0.002
|
|
Z
|
32.93 |
0.013d |
0.021 |
0.006a |
298.65b |
0.022cd |
0.006 |
0.002 |
| S |
35.19 |
Nd |
0.004 |
nd |
189.48b |
0.013d |
0.007 |
0.003
|
|
SPRO
|
32.14 |
0.035c |
0.011 |
0.002b |
330.46b |
0.095ab |
0.010 |
0.002 |
| PRO |
42.10 |
0.069b |
0.015 |
0.002b |
867.01a |
0.146a |
0.009 |
0.002
|
Due to the cooking process of the fufu flour, small amounts of aromatic compounds were found with exception of the acetic acid, which increased during the process of fermentation. Also, the drying process to obtain fufu flour could have driven off the volatiles which give the characteristic offensive odor of traditionally processed fufu. When microorganisms were used by Fagbemi and Ijah20 to enrich fufu, the aroma of the developed product was preferred compared to the commercial fufu after 24 h and 48 h of fermentation. However, no determination of aromatic compounds was done. Dougan et al21 analyzed the aromatic compounds of gari, a fermented cassava based product, before frying. This group found hexanal and nonanal (aldehydes) as we found here in cooked fufu. When Amoa-Awua, Appoh, and Jakobsen22 determined the aroma profile of the uncooked fermented cassava product agbelima, no influence of the type of inocula used during the fermentation process was found. Then, no information of aromatic compounds has been reported after cooking the product as this is commonly eaten in Africa.
Color Evaluation
Lightness (L*) of NF SPRO and LF SPRO differed significantly (p≤0.05) at 0 h of fermentation with the latter having the greater value. There was mainly a predominant influence of fermentation type rather than sample type. At 48 h of fermentation there were significant differences between NFZ and LFZ; and NF SPRO and LF SPRO (p≤0.05). Significant differences in L* were also observed for NFWT between 0 and 96 h fermentation with the latter showing less lightness (p≤0.05). Similar results were visualized for LFWT. NF SPRO presented the greatest mean values for lightness and LF PRO samples at all fermentation times due to the presence of pro-vitamin A that protected the samples from oxidation and loss of lightness. On the other hand, Dziedzoave et al23 observed an overall decrease of yellow color in agbelima, a cassava fermented product, during the course of fermentation. They stated that may be due to the isomerization of the regular carotenoids present in cassava roots at the onset of acid formation during fermentation. Medoua et al24 indicated that a decrease of lightness may be due to browning, caused by the oxidation of phenolics compounds, during fermentation. Predominant sugars such as glucose, fructose, and sucrose may also have an influence on the decrease of lightness. Higher protein flours in the presence of sugars may enhance the Maillard reaction or non-enzymatic browning.25
Regarding a* values, there was a significant effect of sample and fermentation type. Naturally fermented Z, S and S PRO showed significant differences in a* when compared with their analogous inoculated sample (p≤0.05) at 0 h fermentation. Similar results were observed at 96 h of fermentation except for PRO sample. Also, the smallest a* mean value was found for NFZ (-2.16) followed by NFWT (-1.91) at 0 h and NFWT (-2.01) followed by NFZ (-1.82) at 96 h. Consequently, a* values of NF and LF WT and Z were negative (green). On the contrary, S and S PRO were positive (red) and PRO samples values changed from negative to positive (green to red) during fermentation.
Samples with pro-vitamin A, SPRO and PRO, became more yellow with time when the starter culture was not added, as recorded by increases in positive b* values. The nutritive value is largely protected during cooking by the carotenoids insolubility in water. However, they are very sensitive to oxidation, which results in both color loss and destruction of vitamin A activity. In this study, fortified pro-vitamin A cooked samples showed an increase of yellow color over time that suggests lack of detrimental effect of the decrease of pH on color. Even though LFS samples did not contain pro-vitamin A, no significant differences were found in b* values between LFS, LF SPRO and PRO samples at 0 h and 96 h fermentation (p>0.05).
Texture Evaluation
In the case of hardness of the cooked fufu samples, no significant differences were observed between samples within each fermentation time (p>0.05). Therefore, the sample type and addition of the starter culture did not play an apparent role in this attribute.
Regarding adhesiveness, no significant effect of addition of the starter culture was observed at 0 h fermentation (p>0.05). This situation changed when significant differences were observed between all samples at all other fermentation times (p≤0.05). Thus, the composition of the cassava flours significantly influenced the degree of adhesiveness. Even though, similar energy was applied during mixing the cooked fufu samples, it may be possible that mixing intensity could have affected the texture attributes. For example, the breaking up of the gelatinized starch granules in the cooked fufu. The starch leaches out and binds very well with the water added during the pounding process. When the starch granule absorbs water, a good paste is formed and desirable adhesive properties are obtained.
On the other hand, the addition of the starter culture did not influence the adhesiveness of the samples. For example, no significant differences were found on the adhesiveness of the NFWT and LFWT samples at 96 h fermentation (p>0.05). Numfor et al26 compared the textural properties of starch gels from naturally fermented and inoculated fermented cassava starches. Their results showed that the hardness, gumminess, cohesiveness and elasticity of flour gels were reduced in fermented products. Gel hardness and gumminess have been linked both to the degree of granule swelling and network formation by leached amylose. A reduction in cohesiveness of fermented products has been explained as being due to failure of starch granules to release sufficient amylose. The improvement of textural quality has also been attributed to production of organic acids that complex with soluble amylose.
Lastly, only slight differences in springiness were observed between samples within each fermentation time. In most cases, neither sample type nor fermentation type had a significant effect on springiness. For example, there was a significant difference between the springiness of NFZ and LF PRO samples at 0 h fermentation. At 96 h of fermentation, NFZ was significantly different from NF SPRO, LFWT, LFS, LF SPRO and LF PRO (p≤0.05).
SENSORY ANALYSIS
Panelists significantly impacted the perception of all cooked fufu attributes (p≤0.05) (Table 4). There was not a significant effect from sample type, fermentation type, fermentation time and interaction between sample type and fermentation time, fermentation type and fermentation time, interaction between sample type, fermentation type and fermentation time on the fufu descriptor for the aroma attribute (p>0.05). This suggests that using either wild type cassava flour or protein -pro-vitamin A fortified cassava flours, to make a product with a characteristic fufu aroma is feasible.
Table 4: Degrees of freedom and F-ratios from ANOVA results of trained panel evaluation (n=5) for visual appearance, aroma and texture attributes of cooked fufu with and without Lactobacillus plantarum added.
*, **, ***Indicate significant p≤0.05, 0.01, 0.001 respectively.
|
Source of Variation
|
df
|
Visual |
Aroma
|
Texture
|
|
Color intensity for brownness
|
Fermented
|
Stale
|
Fufu
|
Hardness
|
Adhesiveness
|
Springiness
|
|
Panelist
|
4
|
9.59*
|
294.77***
|
121.76***
|
69.39*** |
234.71*** |
15.32** |
261.31***
|
|
Sample Type
|
4
|
134.75***
|
12.65***
|
4.33**
|
1.74 |
15.67*** |
2.89* |
14.03***
|
|
Fermentation Type
|
1
|
26.60***
|
0.34
|
8.72**
|
0.14 |
28.25*** |
7.11** |
27.95***
|
|
FermentationTime
|
4
|
6.40***
|
6.46***
|
1.59
|
1.76 |
4.96*** |
1.99 |
0.26
|
|
Sample Type* Fermentation Type
|
4
|
5.64**
|
1.74
|
0.82
|
4.45** |
3.11* |
5.66*** |
1.16
|
|
Sample Type* FermentationTime
|
16
|
5.63***
|
1.21
|
0.61
|
0.20 |
2.65***
|
1.77*
|
0.51
|
|
FermentationType* FermentationTime
|
4
|
6.29*** |
0.76 |
0.35 |
0.43 |
1.76
|
1.37
|
0.28
|
|
SampleType*Fermentation Type*FermentationTime
|
16 |
3.23*** |
0.78 |
0.52 |
0.67 |
3.44*** |
2.40** |
0.87 |
In addition, no significant effect of fermentation type on fermented aroma was noted. Similarly, no significant effect of fermentation time was found on stale aroma, adhesiveness and springiness texture attributes (p>0.05). However, sample type and fermentation type significantly influenced the perception of texture attributes, hardness, adhesiveness and springiness (p≤0.05). On the other hand, the interaction between fermentation type and fermentation time did not have a significant effect on hardness, adhesiveness and springiness (p>0.05) (Table 4).
Ray and Sivakumar27 found no significant differences in texture, color, odor and overall acceptability of fufu ferment ed from 12 to 96 h. This result disagrees with our findings, where we determined that fermentation time had a significant effect on the brown color intensity, fermented aroma and hardness of cooked fufu (p≤0.05) (Table 4).
The sensory results indicate that cooked fufu is distinctly different when made from different cassava flour materials in terms of all sensory attributes measured except for the fufu aroma attribute. The characteristic odor of traditionally processed fufu has been attributed to the synthesis of organic acids due to certain hetero-fermentative anaerobic bacteria present in fermenting cassava medium.28 However here, addition of L. plantarum did not affect the characteristic fufu aroma. In an acceptability study, Sobowale et al29 determined that the addition of a starter culture strain reduced the characteristic aroma in fufu, thereby enhancing a wider acceptability of fufu as compared to the traditional fufu where no culture was added.
According to Sanni et al2 fufu is considered to be of good quality when it has a smooth texture, characteristic aroma and a creamy-white, grey or yellow color. Based on the color chart used by the panelists, the means color intensity for brownness rating of NFZ and LFZ were lower than the other samples at 0 h of fermentation. This may suggest that Z sample would have a greater acceptability. Thus, Tomlins et al30 determined that fufu flours should be creamier in appearance to increase their acceptability. At 96 hours of fermentation, the LF SPRO showed the highest mean rating for brown color intensity.
Regarding hardness, no significant differences were found between all NF and LF individual samples of a given cassava material, i.e. NFWT versus LFWT (p>0.05) except for SPRO. At 96 h of fermentation, NF SPRO cooked fufu showed the highest mean rating for hardness while NFWT had a significantly smaller mean (p≤0.05). No significant differences were observed for adhesiveness at 72 h and 96 h of fermentation (p>0.05). There were significant differences (p≤0.05) for springiness between NFWT (7.93) and LFWT (10.06) cooked fufu at 72 h of fermentation.
CONCLUSIONS
Lactobacillus plantarum strain 6710 demonstrated its ability to acidify cassava flour during fufu processing. Pasting temperature of the fufu flours established that NFZ (0 h), NFWT/LFWT (96 h) will cook faster than the other samples due to their lower pasting temperature. Fufu flour made from NFZ was more stable as indicated by lowest setback viscosity. Fufu samples generally reached minimum value of pH and maximum acidity at 72 h incubation. Four main aromatic compounds acetic acid, hexanal, nonanal and decanal were detected in most cooked fufu samples at all fermentation times. Color parameters, springiness and adhesiveness texture attributes were most affected by the flour sample type and fermentation time. The sensory results indicated that cooked fufu is distinctly different when made from different cassava flours in terms of all sensory attributes measured. However, the fufu aroma attribute as evaluated by trained sensory panel was not affected by type of cassava flour fermented.
Processing of cooked fufu products with protein and pro-vitamin A fortified cassava flours offer an alternative for fufu consumers; even though a larger acceptability panel would be necessary to indicate the degree of likeness of the developed products.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






