INCREASED PRESCRIPTION AND POTENTIAL ABUSE OF OPIOIDS
Prescription opiates have an important role in alleviation of clinical pain (Table 1).1,2,3,4,5,6 Initially intended for the treatment of acute or terminal pain, opiates are increasingly common in the treatment of chronic non-terminal pain. Consequently, the use and potential abuse of prescription opiates have become widespread, and physicians increasingly encounter patients with a history of opiate use.2,6,7,8,9,10,11,12,13
| Table 1: Equivalent Doses of Different Opioids. |
| Drug |
Oral Route (PO) |
Parenteral Route (IV) |
Conversion Ratio Morphine |
Equivalent Dose of Oral Morphine (mg) |
| Morphine |
30 mg |
10 mg |
IV is 3 times as potent as PO |
30 mg PO |
| Hydrocodone |
20 mg of oral hydrocodone |
N/A |
PO hydrocodone is roughly 1.5 times more potent than OR morphine |
30 mg PO |
| Oxycodone |
20 mg |
N/A |
PO hydrocodone is roughly 1.5 times more potent than PO morphine |
30 mg PO |
| Hydromorphone |
7.5 mg |
1.5 mg |
PO hydromorphone is about 4-7 times as potent as PO morphine.
IV hydromorphone is 20 times as potent as PO morphine |
30 mg PO |
| Fentanyl |
N/A |
15 mcg |
Transdermal fentanyl is
approximately 80 times as potent as morphine |
30 mg PO |
| Meperidine |
300 mg of PO meperidine |
75 mg of IV meperidine |
PO Morphine is about 10 times more potent than PO meperidine and about twice more potent as IV meperidine |
30 mg PO
|
In 2012, health care providers wrote 259 million prescriptions for opioid pain medication, enough for every adult in the United States (US) to have a bottle of pills.14 According, to the Centers for Disease Control and Prevention (CDC), 16,235 people died in the US of overdoses from prescription painkillers or opioids in 2013.15 That figure corresponds to 44 deaths per day. Most of the deaths are among non-Hispanic whites and occur between the ages of 25 and 54. In 2010, 12 million American took opioids for nonmedical purposes, and in 2013 about 2 million people abused or had developed a dependency on opioids. Prescription opioid sales quadrupled in the US between 1999 and 2013.16
In Ohio, for example, 2,482 individuals died in 2014 from an unintentional opioid-related overdose – more than a four-fold increase in 10 years.17 Unintentional opioid overdose has become a leading cause of injury-related death in Ohio over the past decade. Rates of opioid overdose deaths also increased significantly, from 7.9 per 100,000 in 2013 to 9.0 per 100,000 in 2014, a 14% increase, and they were mostly due to opioids such as methadone, oxycodone, hydrocodone, and fentanyl.18
Historically, the CDC has programmatically characterized all opioid analgesic-related deaths (from natural and semisynthetic opioids, methadone, and other synthetic opioids) as “prescription” opioid overdoses.19 Rates of opioid overdose deaths increased from 7.9 per 100,000 in 2013 to 9.0 per 100,000 in 2014, a 14% rise. Between 2013 and 2014, the age-adjusted rate of death involving methadone remained unchanged. However, the age-adjusted rate of death involving natural and semi-synthetic opioid pain relievers, heroin, and synthetic opioids other than methadone (such as fentanyl) increased by 9%, 26%, and 80%, respectively. The opioid overdose epidemic continues to worsen. The sharp increase in deaths involving synthetic opioids (other than methadone) in 2014 coincided with law enforcement reports of increased availability of illicitly manufactured fentanyl. However, illicitly manufactured fentanyl cannot be distinguished from prescription fentanyl in death certificate data. In 2014, 61% (28,647) of drug overdose deaths involved some type of opioid, including heroin. The age-adjusted rate of drug overdose deaths involving opioids increased significantly from 2000 to 2014, increasing 14% from 2013 (7.9 per 100,000) to 2014 (9.0 per 100,000) (Figure 1).20 There are urgent needs for redoubled action to prevent opioid abuse, dependence, and death. There should be improved treatment capacity for opioid use disorders and reduction of the supply of illicit opioids, particularly heroin and illicit fentanyl.
Figure 1: Age-Adjusted Rate of Drug Overdose Deaths and Drug Overdose Deaths Involving Opioids – United States, 2000-2014. This Chart is from Rudd et al of the Division of Unintentional Injury Prevention, US National Center for Injury Prevention and Control, CDC.20
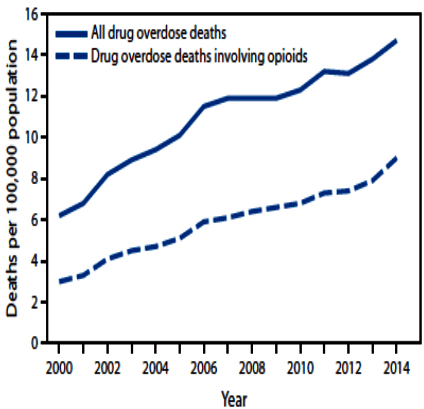
From 2013 to 2014, the largest increase in the rate of drug overdose deaths involved synthetic opioids other than methadone (e.g., fentanyl and tramadol), which nearly doubled from 1.0 per 100,000 to 1.8 per 100,000 (Figure 2).20 Heroin overdose death rates increased by 26% from 2013 to 2014 and have more than tripled since 2010, from 1.0 per 100,000 in 2010 to 3.4 per 100,000 in 2014 (Figure 2).20 In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (such as morphine, oxycodone, and hydrocodone), 3.8 per 100,000, was the highest among opioid overdose deaths, and it increased 9% from 3.5 per 100,000 in 2013. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in both 2013 and 2014.18
Figure 2: Drug Overdose Deaths Involving Opioids, by Type of Opioids – United States, 2000-2014. This Chart is from Rudd et al of the Division of Unintentional Injury Prevention, US National Center for Injury Prevention and Control, CDC.20
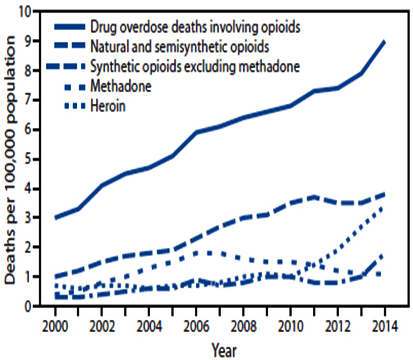
In Massachusetts, there has been a three-fold increase in opioid-related overdose deaths from 2000 to 2015, and nearly 1300 residents across the Commonwealth died from overdose in 2014. In 2014, Massachusetts was among the top ten states for prescribing prescription opioids, with 4.6 million Schedule II and III medications prescribed for a total of 255 million pills. The rising prevalence of prescription opioids across the country has been associated with the increased prevalence of opioid use disorder and overdose death.18,19,20
At Massachusetts General Hospital Boston, MA, USA, an estimated 250,000 to 300,000 ambulatory opioid prescriptions are written each year. Based on a conservative estimate that each prescription dispenses an average of 30 pills, that hospital annually prescribes more than 7.5 million of opioid pills in the outpatient setting alone.
APPROACH TO POST-OPERATIVE PAIN MANAGEMENT IN NON-OPIOID USERS AND CHRONIC OPIOID USERS
The global epidemic of opiate use continues to spread, especially in developing countries.21 The tolerance to opioids may develop as quickly as 2 weeks after beginning treatment.22 The post-operative pain control is challenging in opioid dependent patients. Some clinical studies22,23,24 reported that patients who chronically consumed opioids before surgery experienced increased post-operative pain despite receiving a 3- to 4-fold greater amount of opioid than patients who were considered opioid-naive.
More than 80% of patients who undergo surgical procedures experience acute post-operative pain, and approximately 75% of those with post-operative pain report the severity as moderate, severe, or extreme.24,25 Less than half of patients who undergo surgery report adequate post-operative pain relief.24 Inadequately controlled pain negatively affects quality of life (QoL), function, functional recovery, the risk of post-surgical complications, and the risk of persistent postsurgical pain.26 Many pre-operative, intra-operative, and post-operative interventions and management strategies are available and continue to evolve for reducing and managing post-operative pain.
Pre-operative Education and Peri-operative
Individually tailored programs of education and support for surgical patients with more intensive needs (due to medical or psychological comorbidities or social factors) are associated with benefits including reduced post-operative opioid consumption,27,28 less pre-operative anxiety,29,30,31,32 fewer requests for sedative medications,28 and reduced length of stay after surgery.27,31,33,34 Although, the optimal timing and content of pre-operative education is uncertain, pre-operative education routinely includes discussion of changes in use of analgesics before surgery (e.g., discontinuation of aspirin for procedures in which hemorrhage would present high risks or in patients at high risk of hemorrhage) and continuation of medications (such as opioids, benzodiazepines, gabapentinoids, or baclofen) to avoid a withdrawal syndrome, unless there is a specific plan to taper. Education should also correct any underlying misperceptions about pain and analgesics. Misperceptions might include beliefs that pain after surgery does not warrant treatment, that healthcare providers will only respond to extreme expressions of pain, that opioids are always required for post-operative pain, or that opioid use inevitably leads to addiction.35 Pregnant women who undergo surgery should be informed about potential effects of treatment options on the fetus and the breastfed newborn.36
Pain Assessment and Indications
In post-operative situations, opioid prescriptions should be based on the degree of tissue disruption, a strong consideration of alternatives, specialty specific published guidelines, the impact of pain upon function, and the risk/benefit ratio given the provider’s knowledge of the individual patient. Opioids should only be prescribed after a clinical examination, diagnosis, review of medication and medical/psychiatric history, consideration of alternatives, and review of data from the prescription monitoring program (PMP).
Moreover, more and more patients are chronic opioid users in the pre-operative period. Chronic opioid use in the pre-operative period may have a negative impact on outcomes following surgery. Zywiel et al37 demonstrated a higher risk of complications and prolonged painful recoveries in chronic opioid users undergoing total knee arthroplasty (TKA). Lawrence et al38 reported worse outcomes in chronic opioid users undergoing anterior cervical diskectomy and fusion. These findings underscore the importance of incorporating opioid use assessment as a routine part of the pre-operative evaluation.
Pain assessment and reassessment are required to provide optimal post-operative pain care. Pain assessment helps determine whether pain management is adequate, whether analgesic or analgesic dose changes are required, whether changes in the post-operative pain management plan or additional interventions are warranted, and (in the case of difficult to manage pain) whether specialty consultation or other measures are needed. Because pain is inherently subjective, patient self-report is the primary basis of all pain assessments.39,40
Assessment should determine what interventions have been effective for the pain, how the pain affects function, the type of pain (neuropathic, visceral, somatic, or muscle spastic), and whether there are barriers to effective pain management, such as cultural or language differences, cognitive deficits, or patient misconceptions about pain management.
INTERVENTIONS IN NON-OPIOID USERS
Non-Opioid Alternatives to Pain Management
Optimized therapy for co-morbid illnesses: Problems include sleep problems, depression, and anxiety. Those conditions increase severity of pain and the need for opioids.
Non-pharmacologic treatment: Non-pharmacologic therapies should be considered as a primary therapy for post-operative pain unless the natural history of the cause of pain or clinical judgment warrants a different approach. These therapies often reduce pain with fewer side effects and can be used in combination with non-opioid medications to increase likelihood of success. Examples include, but are not limited to: (1) Ice, heat, positioning, bracing, wrapping, splints, stretching and directed exercise often available through physical therapy; (2) Massage therapy, tactile stimulation, acupuncture/acupressure, chiropractic adjustment, manipulation, and osteopathic neuromuscular care; (3) Biofeedback and hypnotherapy.
Non-opioid pharmacologic treatment: Non-opioid medications should be used along with non-pharmacologic therapy. When initiating pharmacologic therapy, patients should be informed as to proper use of medication, importance of maintaining other therapies and expectation for duration and degree of symptom improvement. Treatment options, by the quality of pain, are listed below: nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentin, clonidine and topical lidocaine.
Regional techniques: (1) Consider consultation with a pain specialist. (2) Continuous peripheral nerve blocks or continuous epidural anesthesia are far superior to systemic opioids in managing post-operative pain.
Opioid Pharmacologic Treatment
Short-term opioid therapy may be preferred as a first line therapy in the immediate post-operative period. In most cases, opioids should be used as adjuncts to additional therapies, rather than in isolation.41 It is critical that healthcare providers communicate with one another about a patient’s care if the patient may be receiving opiate prescriptions from more than one provider.
The following are recommendations for the general use of opioids to manage post-operative pain: (1) Appropriate risk screening should be completed (e.g., age, pregnancy, high-risk psychosocial environment, personal or family history of substance use disorder); (2) Provide the patient with the least potent opioid to effectively manage pain. A morphine equivalence chart should be used if needed. (3) Prescribe the minimum quantity needed with no refills based on each individual patient, rather than a default number of pills. (4) Consider checking PMP for all patients who will receive an opiate prescription. (5) Avoid long-acting opioids (e.g., methadone, oxycodone ER, and transdermal fentanyl). (6) Use caution when prescribing opioids to patients receiving other medications causing central nervous system (CNS) depression (e.g., benzodiazepines and sedative hypnotics) or patients known to use alcohol. Depressant combinations increase the risk of respiratory depression and death. (7) Discuss with the patient a planned wean of opioid therapy, concomitant with reduction or resolution of pain. (8) Prevent complications/abuse and discuss proper secure storage and disposal of unused medication to reduce risks to the patient and others. Patients should be made aware of the risk of pills being potentially used by family members, roommates, children, or strangers. Recommend that patients store any controlled substances in locked containers/cabinets and away from other household medications. (9) Remind the patient that it is both unsafe and unlawful to give away or sell opioid medication, including unused or leftover medication.
Special populations: If a patient is currently on long-term opioid medications or opioid agonists/antagonists, any interruption in medications in the acute setting must be discussed with the prescribing clinician. The initial prescribing clinician of these medications should anticipate communications from providers in the acute settings and provide the appropriate guidance and/or support.
Reassessment of pain and follow-up: Re-evaluation of patients who receive opioid therapy for post-operative pain will be considered if opioid therapy will continue beyond 14 days. This reevaluation may be through an office visit or phone call based on the discretion of the provider. If opioids are prescribed for post-operative pain, close follow-up should be arranged with the primary surgeon/prescribing clinician and the primary care provider. Earlier follow-up should be considered for patients with one or more of the following risk factors: (1) Chronic opioid use. (2) History of prior substance use disorder. (3) Psychiatric or medical conditions increasing risk of opioid related problems. (4) Current use of other medication potentiating risk of opioids such as benzodiazepines. (5) If the patient requires additional opioids prior to scheduled follow-up the patient should present for evaluation of complication or other possible cause of increased pain. Only after all reasonable and safe alternatives to long-term use of opioid medications have been considered and exhausted should chronic use of opioid analgesia be considered. Chronic opioid therapy (COT) is rarely the best approach for the treatment of chronic pain. At all follow-up visits, ask about any unused medication, discuss the importance of safe disposal, and provide safe medication disposal flyer.
Provider monitoring: Regular opioid prescribing reporting helps prescribers and clinical leaders to identify opportunities to improve prescribing practices, including: (1) Assessing adherence to best practices, guidelines and policies. (2) Benchmarking prescribing rates and exploring inter-prescriber variations. (3) Reviewing utilization to detect and secure against diversion.
INTERVENTIONS IN CHRONIC OPIOID USERS
Pre-operative Considerations
Pre-operative visits with a patient provide opportunities to discuss the risks of chronic opioid use and to address his or her concerns regarding post-operative pain control. Early discussions about pain-related beliefs in the pre-operative period may facilitate a more adaptive response to post-operative pain.42
Peri-operative Interventions
A multimodal approach combining analgesics with non-opioids treatment and regional anesthesia throughout the peri-operative period should be strongly considered in intervertions for post-operative pain control in opioid tolerant patients. Sinatra et al43 provide a comprehensive review on the role of opioids, non-opioids, and nerve blockade in orthopaedic patients. The choice depends on the strategy favored by the physician and the availability of drugs and equipment.44
Pre-emptive Analgesia
Pre-emptive analgesia is the administration of pain medication in the pre-operative period to exert a preventive effect against post-operative pain by inhibiting central autonomic hyperactivity. The route and type of agents used can be chosen from any number of permutations and include regional anesthesia, nonsteroidal anti-inflammatory drugs, opioids, anticonvulsants, and acetaminophen. The goal of multimodal analgesia is to reduce opioid consumption.
N-methyl-D-aspartate (NMDA) antagonism: Ketamine provides intense analgesia by blocking N-methyl-D-aspartate (NMDA) receptors and thereby reducing the release of the excitatory neurotransmitter glutamate. An intravenous dose of 0.5 mg/kg ketamine on induction with a continuous infusion of 10 mcg/kg/min has been shown to reduce opioid consumption and pain scores for up to 6 weeks in opioid-dependent patients undergoing back surgery for chronic back pain.45
Alpha 2 agonists: Dexmedetomidine is a selective alpha 2 agonist with both analgesic and sedative properties. When combined with opioids in intravenous patient-controlled analgesia, the presence of dexmedetomidine demonstrated lower post-operative pain intensity and lower post-operative morphine-equivalent opioid consumption during the first 12 or 24 hours.46 Tizanidine is an alpha 2 – adrenoceptor agonist with potent muscle relaxant activity. It has been showed decreased pain intensity and decreased need for opioid analgesics.47
Gabapentinoid: Gabapentinoids are analogs of γ-aminobutyric acid. They can bind to the α-2δ subunit of presynaptic calcium channels and therefore modulate glutamate expression. These drugs may inhibit pain transmission and central sentization.48,49
Anti-inflammatory Medication: The analgesic effects of acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) have been attributed to their anti-inflammatory properties through inhibition of prostaglandin synthesis. Selective COX-2 inhibitors seem to have an advantage in acute pain management.50
Local Anesthetics: Lidocaine demonstrates analgesic and anti-inflammatory effects through acting on potassium channels, calcium channels and protein G-coupled receptors51 and significantly lower post-operative pain score visual analog scales (VAS) at 1-4 hours and 24 hours after surgery, but not 48 h.52
Beta-blocker: Esmolol is one of the short-acting cardioselective β-1 adrenergic receptor antagonist with a distribution half-life of around 2 minutes and an elimination half-time of around 9 minutes.53 Some studies have found that esmolol reduces anesthetic requirements and decreases the use of peri-operative opioids.54,55
Regional Anesthesia
Regional anesthesia can improve pain control, decrease systemic opioid requirements, and reduces risks of respiratory complications and venous thromboembolism.43,44,56,57 Regional anesthesia can involve neuraxial (central) or peripheral blockade. In general, central neuraxial blocks alone or in combination with catheter techniques provide better pain control.58 Peripheral nerve blocks under the guidance of ultrasound seem to lack systemic side effect, lesser incidence of minor complications, and reduce the opioids requirements.59
CONCLUSION
Pharmaceutical options and various regional blocks are available to help to control post-operative pain, and a multimodal pain management approach may be particularly beneficial in chronic opioid users undergoing surgery.
These strategies above are based on a premise that optimal management begins in the pre-operative period. After assessment of the patient, a plan of care should be generated to the individual and to the surgical procedure, with follow-up assessments and adjustments. Further research and observation needed on practice gaps regarding use of evidence-based interventions for management post-operative pain.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







