INTRODUCTION
Penile cancer is a rare primary malignant tumor.1 In Europe and the United States, the incidence of penile cancer was estimated at 1 case per 100,0001,2,3 and varied depending on geographic area.2,3 Several studies carried out in Africa, including Senegal, have confirmed the rarity of this condition, and the diagnosis is often made at an advanced stage of the disease, requiring radical treatment often refused by patients due to religious beliefs and taboos.4,5,6,7 The purpose of this study was to present the epidemiological, diagnostic, and therapeutic characteristics of patients with penile cancer in the region of Thies, Senegal.
PATIENTS AND METHODS
We carried out a retrospective descriptive study between January 2015 and December 2020 in the different hospitals in the Thies region: the public hospitals (Entidades Promotoras de Salud (EPS)) of Thies, Mbour, Tivaouane, and Saint Jean Dieu hospital (public/private hospital). We included all patients followed for penile cancer whose pathological examination confirmed the diagnosis from biopsy samples or surgical specimens. For each patient, the following parameters were studied: the age at the time of diagnosis, the time between the appearance of the lesion and the first consultation, the existence of risk factors or precancerous lesions, the extent of the disease and tumor size, pathologic type, therapeutic modalities, and follow-up after treatment. Histological examination of biopsy fragments of the lesion and/or the surgical specimen confirmed the diagnosis. An extension assessment was carried out, and then the staging was obtained according to the Tumour, Node, Metastasis (TNM) 2016 classification. Our patients had monthly and then quarterly clinical monitoring during the first year and biannually thereafter for the evaluation of recurrence or progression of metastatic disease. Written informed consent was obtained from all patients for the use of their anonymized clinical data.
RESULTS
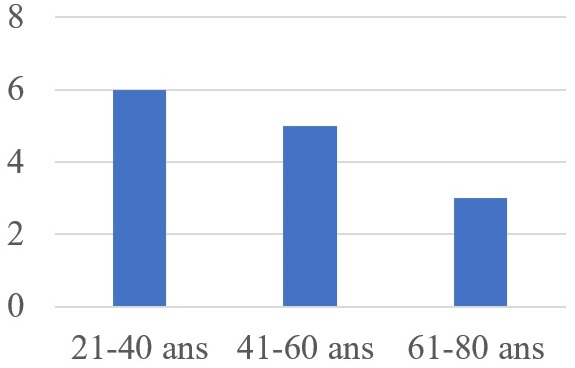
During the study period, out of 478 cases of urogenital cancer, 14 cases of penile cancer were treated in different hospitals in the Thies region (Figure 1), representing an annual frequency of 2.2 cases per year and 2.9% of all cases of urogenital cancer. During the study period, the male population of the Thies region was estimated at 1,172,603. The mean age of the patients was 42.93-years (range: 26 to 82 years). All patients were circumcised during childhood. The age group 21 to 40-years was the most common (Figure 2). They all had low socio-economic status. We noted papilloma virus infection in 14.2% of patients. The average consultation time was 18-months (range: 5 to 48-months). The main reasons for referral were penile pain (64.3%), urinary disorders of the lower urinary tract (35.7%), penile swelling (14.3%), and deterioration of general status (Table 1). Physical examination revealed a tumor confined to the glans or located in the distal third of the penis in 57.2% of cases, or occupying the entire penis in 42.8%. It was an ulcerative-vegetative tumor in 57.2%, ulcerated in patients (28.5%), infiltrating (14.2%), with only one patient presenting with a vegetating cauliflower tumor limited to the glans (Table 1). The presence of palpable bilateral superficial inguinal lymphadenopathy was noted in 35.7% of patients. One of the patients presented with vitiligo affecting the penis and the scrotum (Figure 3).
Figure 1. Distribution of the Patients According the Hospital
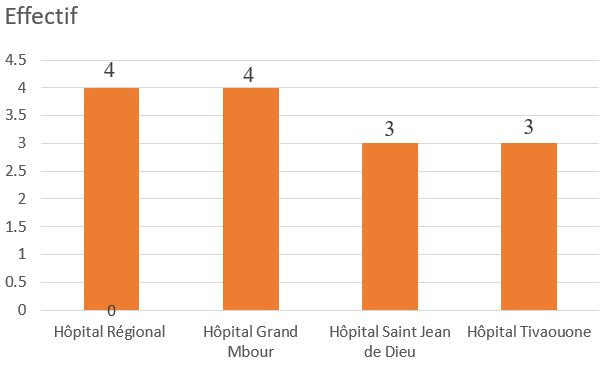
Figure 2. Distribution of Patients according Age Range
| Table 1. Distribution of Patients According Epidemiological. Diagnosis and Therapeutic Apects |
| Variables |
Effective |
Percentage |
| Mean age=42.93 years (range 22 and 67 years) |
| Marital status |
Bachelor |
4 |
28.5% |
| Married |
5 |
35.75% |
| Divorce |
5 |
35.75% |
| Risk factors |
HPV infection |
2 |
14.3% |
| Phimosis tigh |
2 |
14.3% |
| Average Consultation time=18 months |
| Clinical presentation |
Penile pain |
9 |
64.3% |
| Penile swelling |
4 |
28.6% |
| Urinary disorders |
5 |
35.7% |
| Urethrorrhagia |
2 |
14.3% |
| General condition alteration |
10 |
71.4% |
| Physical Examination |
| Tumor Topography |
| Glans≤1/3 Distal Penile (cavernous) |
|
8 |
57.2% |
| Glans+penile shaft |
|
6 |
42.8% |
| Midsized of tumour=(range size 2 cm-8 cm) |
Infiltrating |
2 |
14.3% |
| Ulceration |
4 |
28.6% |
| Ulcero-Budding |
8 |
57.1% |
| Inguinal lymphadenopathy |
5 |
35.7% |
| Histological Tumour Type |
| Squamous cell carcinoma |
14 |
100% |
| Histological grading |
G1 |
2 |
14.3 |
| G2 |
5 |
35.7 |
| G3 |
1 |
7.1 |
| G4 |
6 |
42.9 |
| Treatment |
Partial Penectomy |
7 |
50% |
| Total Penectomy |
6 |
42.8% |
| Radical inginal lymphadenectomy |
5 |
35.7% |
| Chemotherapy |
1 |
7.1% |
| Refused treatment |
1 |
7.1% |
| Average Length of hospitalization=2.4 days |
Figure 3. Squamous Cell Carcinoma Aspect
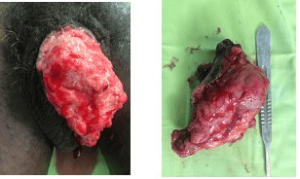
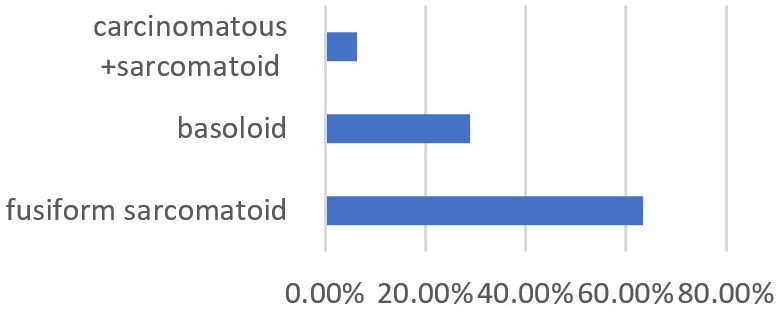
Pathology examination concluded that there was invasive squamous cell carcinoma in all cases (100%). The carcinoma was spindle-shaped sarcomatous in 64.3% of cases, basaloid in (28.6%), and carcinomatous with sarcomatous components in 7.1%) of patients (Figure 4). According to the TNM 2016 classification, the carcinoma was classified as G1 in 2 cases, G2 in 5 patients, G3 in 1 case and G4 in 7 patients. The Table 2 shows the distribution of patients according to epidemiological, diagnosis, and therapeutic aspects.
Figure 4. Distribution of Patients according Histological Type of Squamous Cell Carcinoma
| Table 2. Distribution of Patients According Evolution |
| Evolution |
Effective |
Percentage |
| The overall 3-year survival |
3 |
21.4% |
| Death |
5 |
35.7% |
| Recurrence with tumor progression |
2 |
14.3% |
| Lost to follow-up |
4 |
28.5% |
| Total |
14 |
100% |
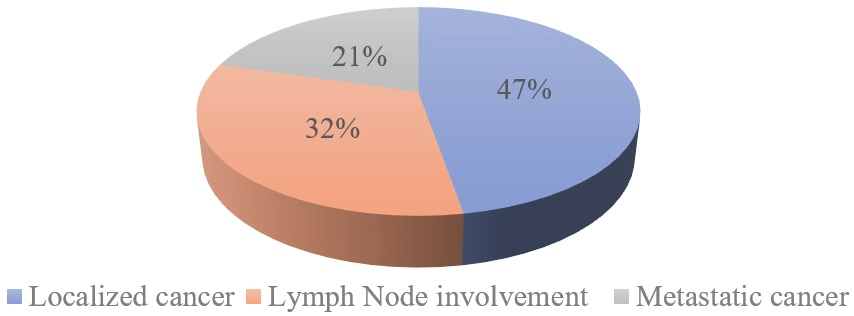
At the end of the extension assessment, 47.3% of patients presented a localized tumor, and 32.3% of patients had lymph node metastases (Figure 5). A partial amputation of the penis was performed in 50% of patients, and a total amputation was performed in 42.8% of patients associated with bilateral lymph node dissection in 35.7%. One patient refused total penile amputation (Table 1). One patient classified as T2N0M0 G3 received adjuvant chemotherapy after the surgery. The mean postoperative follow-up duration was 2.5 years (range: 6 months to 5-years). Local recurrence with tumor progression was observed in two patients (14.3%) 6-months after total amputation of the penis. Five patients (35.7%) died before additional treatment. The overall 3-year survival was 21.4% for operated patients. Those lost to follow-up represented 28.5% (Table 2).
Figure 5. Distribution of Patients according Stage
DISCUSSION
Epidemiology
With an overall incidence of around 1/100,000 males in Europe and the USA, penile cancer is uncommon.3 In the USA, the highest incidence is among white Hispanics (1.01), followed by Alaskans and Native American Indians (0.77), African Americans (0.62), and white non-Hispanics (0.51) per 100,000, respectively.1,2,3
Penile cancer is a rare tumor in Senegal.4,5,6 Gueye et al4 estimated its frequency at 0.35% of all cancers and 0.97% of adult male cancers in 1992. Sow et al5 reported 8 cases in 12 years. In some countries in Africa, South America, and Asia, the incidence of penile cancer can reach 10% of cancers in men. In Kenya, 31 cases were reported in 20 years by Magoha et al.8 In Brazil, Luciano et al9 reported an incidence of 2.9-6.8 cases per 100,000 men. In a retrospective study, Chalya et al10 in Tanzania reported 236 patients with penile cancer.
The average age of patients in our series was 42.9-years. The mean age was 47-years in the study of Chalya et al10 and 47.9-years in that of Magoha et al.8 In Europe, the incidence of penile cancer increases with age, and it is most often a cancer in men aged over 50-years.11,12 The peak occurrence is in the 6th decade, but penile cancer does occur in younger men.13 Several risk factors involved in the occurrence of penile cancer11,12,13,14 have been identified. Human papillomavirus (HPV) deoxyribonucleic acid (DNA) was believed to be involved in 70-100% of intraepithelial neoplasia.
HPV-16 and HPV-18 are two risk factors found in 50% of cases of penile cancer.11,12,13,14,15,16 The HPV virus interacts by activating oncogenes and inhibiting tumor suppressor genes (p16, P53 and Rb genes).17,18 HPV action may explain the variations in incidence depending on the region. In the literature, other risk factors, such as poor hygiene and premalignant penile lesions (atrophic lichen sclerosus, condylomas, Querat’s erythroplasia, and Bowen’s disease), have been identified as being able to promote the occurrence of penile cancer.16 On the other hand, in the opinion of certain authors, neonatal circumcision seems to have a protective role.4,5,8 In Senegal, circumcision is widespread during childhood.
Diagnostic Aspects
In our series, 64.3% of patients consulted for pain localized to the penis; urinary symptoms were present in 35.7% of patients. This reflects a late presentation of the disease, as in most African publications, where the diagnosis is often made at an advanced stage due to modesty, taboos, and religious beliefs.2,3,4,5,15 On the other hand, in Western series, it is a lesion of the penis, most often painless, with a budding (vegetative) or ulcerated appearance, affecting the glans or the foreskin in 95% of cases.11,12,13,14 The lesions can be hidden under phimosis.11 Luciano et al7 found 73% of lesions were localized at the glans and foreskin. Squamous cell carcinoma is the main histological type encountered in our series. There are two types: ulcero-infiltrative tumors and exophytic (papillary) tumors. Its locoregional extension, for some authors, is via the lymphatic route towards the inguinal lymph nodes.14 Basaloid carcinoma is an ulcerated, non-exophytic tumor with a vertical pattern of extension.14 For basaloid-type squamous cell carcinoma, some authors estimate lymphatic extensions would be frequent with a higher grade and high metastatic risk.14,15,19
Treatment
According to recommendations3,14 for superficial lesions of the penis, photodynamic treatment, cryosurgery with liquid nitrogen, and topical 5-fluorouracil combined with biopsy have been used successfully.16,17 In Africa, the usual diagnostic delay requires us to resort to partial or total amputation of the penis.2,3,4,5 These mutilating procedures can have a psychological and negative impact on the quality of sexual life. This sometimes poses a problem of the acceptability of penile amputation, as reported by several authors.4,5,6 Total inguinal lymphadenectomy consists of the removal of all superficial and deep inguinal lymph nodes.3,4,16 In the literature, morbidity after lymph node dissection would be estimated between 30 and 70% with complications such as lymphocele, skin necrosis, parietal infection, hemorrhagic disease, and lymphedema.14,20
In cases of fixed inguinal lymph node invasion, the objective of neoadjuvant chemotherapy is to allow tumor reduction and facilitate excision.14 The TPF-type protocol (paclitaxel, cisplatin, and 5-fluorouracil) to treat noperable tumors or to treat lymph node recurrence. Half of the patients were alive without recurrence after more than 25-months.
After bilateral total inguinal dissection, Hakenberg et al3 obtained good results in patients receiving chemotherapy combining bleomycin, vincristine and methotrexate after a follow-up period of 42 months on average.
Adjuvant chemotherapy would be preferable to adjuvant radiotherapy.14 Radiotherapy is not recommended to treat lymph node metastases from penile tumors. Adjuvant radiotherapy would be an option to treat cN3 patients or in patients receiving palliative treatment for surgically inextirpable lymphadenopathy.
In the Thies region, there is no radiotherapy and chemotherapy center. Patients are sent to a single center in Dakar (Senegal) to benefit from additional treatment where treatment times are often long. It is therefore appropriate to offer psychological support to these patients over the long-term and to raise the medical technical level while promoting multidisciplinary collaboration through consultation meetings.
The Prognosis
The prognosis of penile cancers depends on the TNM stage, histological type, tumor grade, and the existence of vascular and lymphatic invasion.24,25,26 The proposed monitoring schedule depends on the risk of the penile tumor and lymph node status.3,26 Some authors estimated that in patients without lymph node metastasis, the 5-year survival rate was 80% and approximately 50% when lymph nodes were involved.5,7,26 This survival rate was less than 30% in the case of metastases.5,7,26 Metastasis often occurs in lymph nodes, the brain, and the lungs.
CONCLUSION
Cancer of the penis is a rare tumor in the Thies region of Senegal and poses a therapeutic management problem due to diagnostic delay. Surgical excision is the standard treatment.
FUNDING
None.
ETHICAL CONSIDERATIONS
The authors declare having obtained the consent of the patients regarding the use of the data.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.










