INTRODUCTION
Infectious bursal disease is an acute and highly contagious disease which is mostly affects young chickens of 3-6 weeks old. The disease, also named “Gumboro disease” according to the location of the first outbreaks in Gumboro, Delaware, USA, was initially described as avian nephrosis due to damage seen in the kidneys,1 but was later designated infectious bursal disease (IBD) according to varying morphologic and histological changes observed in the bursa of Fabricius.2
The causal agent of IBD is infectious bursal disease virus (IBDV), which is a non-enveloped double stranded RNA (dsRNA) virus belonging to the genus Avibirnavirus, family Birnaviridae.3,4,5 Infectious bursal disease virus genome has two double-stranded RNA segments; these are segment A and segment B.6 Segment A contains two partially overlapping open reading frames (ORFs), these are; ORF1 and ORF2. The small ORF1 encodes a non-structural protein VP5, whereas the large ORF2 encodes a precursor polyprotein (NH2-VP2- VP4-VP3-COOH), which is processed to produce mature protein like VP2, VP3 and VP4. The largest ORF contains a polyprotein that is proteolytically cleaved to form three polypeptides: namely VP2 and VP3 are the structural proteins, whereas VP4 is a protease.3,7 Segment B contains one ORF encoding VP1,7,8 with the multifunctional protein having the polymerase (an RNA-dependent RNA polymerase) that is responsible for viral genome replication, mRNA synthesis and capping enzymatic activity.9
The IBDV is one of the most economically important diseases that affect the growth of young chickens which results in significant economic losses in the poultry industry.10 The virus has a selective tropism for actively dividing bursal B-lymphocytes which leads to massive destruction of B-lymphocytes in the bursa and to a lesser degree, in other lymphoid organs thereby causing prolonged immunosuppression of chickens infected before 3 weeks of age.11,12
Commonly young chickens at 0-2 weeks old have a high level of maternally derived anti bodies (MDA) hence, resistance to IBD. Nevertheless, the MDA level declines with age and so also bursa of Fabricius. Once the target organ reaches its maximum development between 3 to 6 weeks, thereafter the chickens will be highly susceptible to IBD.13
Strains of IBDV can be grouped within two distinct serotypes by virus neutralization test.14 Serotype 1 is pathogenic in chickens and serotype 2, isolated from turkeys, is not pathogenic in chickens or turkeys.6 Serotype 1 strains are further classified into very virulent (VV), classical virulent, and sub-clinical on the basis of pathogenicity.15 Very virulent strains of serotype 1 IBDV are common worldwide and cause serious disease. Clinical IBD can be diagnosed by a combination of characteristic signs and post-mortem lesions. Subclinical IBD can be confirmed in the laboratory by demonstrating a humoral immune response in unvaccinated chickens, or by detecting viral antigens or viral genome in tissues. In the absence of such tests, histological examination of bursae may be helpful.16
Clinical IBD is responsible for heavy economic losses due to impaired growth, death and also from the excessive condemnation of carcasses because of skeletal muscle haemorrhages17,18 and losses due to immunosuppression.19 The clinical features of the IBD included whitish or watery diarrhoea, followed by anorexia, depression, trembling, severe prostration, and death. At necropsy, the birds exhibited dehydration, haemorrhages in the leg and thigh muscles, urate deposits in kidneys and enlargement of the bursa of Fabricius.20 Infectious bursal disease has not been reported to have any zoonotic potential.21
The infection, when not fatal, cause immunosuppression, in most cases temporary, depending on the virulence of the strain, the age, breed and the presence or absence of passive immunity. In chicken flocks, clinical picture and the course of the disease usually are indicative of an IBDV infection, and the pathological changes observed at the Bursa of fabricius (BF) are characteristic.13 Presence of this disease causes alterations in different haematological and serum biochemical parameters in poultry.18 The immunosuppressive effects of IBDV infections enhance the chicken’s susceptibility to secondary bacterial infections such as gangrenous dermatitis, Escherichia coli infections, with a viral infection like chicken anemia agent, inclusion body hepatitis, respiratory diseases.11
Therefore, the objective of this paper is to highlight various commonly used diagnostic techniques of IBD.
DIAGNOSIS OF INFECTIOUS BURSAL DISEASE
Infectious Bursal Disease is diagnosed by considering the flock’s history and of the clinical signs and post mortem lesions. Obviously, chickens less than 3 weeks of age present no clinical signs of disease but chickens greater than 3 weeks of age present clinical signs.22 Clinical manifestations and postmortem findings of affected birds may aid to diagnose a disease but laboratory diagnosis is necessary for confirmation of the diseases.23
Isolation and identification of the agent provide the most certain diagnosis of IBD but are not usually attempted for routine diagnostic purposes because the virus is difficult to isolate. In practice, laboratory diagnosis of IBD depends on detection of specific antibodies to the virus, or on detection of the virus antigen and nucleic acid in tissues, using immunological or molecular methods.16 Confirmatory diagnosis of IBDV is most commonly performed by serology using Enzyme linked immunosorbent assay (ELISA), Agar gel precipitin test (AGPT) and Virus neutralization test (VNT) of bursal sections.24
Clinical Signs
Infectious bursal disease virus has a short incubation period of 2-3 days and the infection generally last 5-7 days. One of the earliest sign of IBDV infection is the tendency for the bird to engage in vent picking.25
In a typical outbreak in 2- to 15-week-old chickens, some 10-20% of the flock may show sudden signs. Observing any signs is difficult during the very early stages. One of the earliest signs is whitish or watery diarrhoea, with vent feathers soiled by urinary material. This is followed by anorexia, depression, trembling, severe prostration, and death.26 The disease is manifested by debilitation, dehydration and development of depression with the swollen and bloodstained vent.27
Differential Diagnosis
The lesions and symptoms of coccidiosis are very similar to IBD. Similar to IBD in coccidiosis there are sudden onset, ruffled feathers bloody droppings but no bursal lesion.28 However, muscular haemorrhages and edema of bursa differentiate IBD from coccodiosis. Other diseases that resemble IBD are infectious bronchitis virus, haemorrhagic syndrome, Marek’s disease.29
Quinn reported that if there are enlarged muscular haemorrhages and enlarged edematous or haemorrhagic cloacal bursas, it would suggest IBD, however, the involvement of cloacal bursa usually will distinguish IBD from other nephrosis causing condition. Marek’s (causes bursal atrophy, but nerve lesion is very distinct and also marek’s forms tumors). Haemorrhages syndrome (cause bursal muscular mucosal haemorrhage, but with no bursal lesion) is the usual manifestation of the diseases.28
Non-infectious disease like mycotoxins are causes of bursal atrophy.30 In aflatoxins there is a degeneration of both thymus and the bursa and trichothecenes generally depress lymphocytopoesis. High doses of zearalenon also cause decreased bursal weight. As in other species, steroidal anti inflammatory drug like, corticosteroids cause apoptosis and decrease the production of lymphocytes and smaller bursas can be occurred in animals that are in poor nutritional states or stressed from other reasons.31,32
Gross Lesions
Changes in lymphoid organs are typical of the disease. The BF, which is the main target of the virus, undergoes major changes beginning at 3 d post-infection (PI) when it increases in size reaching twice the normal size by 4 d PI followed by atrophy, reaching one third of its original weight by 8 d PI. The increase in size is accompanied by a red coloration.33 Autopsies performed on birds that died during the acute phase (three to four days following infection) indicate hypertrophic, hyperaemic and oedematous bursas. The most severe cases are characterized by a major infection of the mucous membrane and a serous transudate, giving the bursal surface a yellowish colour. This appearance is often accompanied by petechial and haemorrhages. The affected birds are severely dehydrated, and many birds have hypertrophic and whitish kidneys containing deposits of urate crystals and cell debris.34
Haemorrhages in the pectoral muscles and thighs are frequently observed, probably due to a coagulation disorder.35 Office International des Epizootics16 stated that the bursae of chickens infected with virulent serotype 1 IBDV appeared yellowish (sometimes haemorrhagic) with black cherry appearance and turgid, with prominent striations (Figures 1 and 2).
Figure 1. Haemorrhages on Thigh Muscles and Breast Muscles36
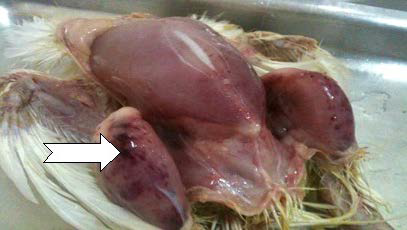
Figure 2. The Presence of Haemorrhages on the Serosal Surface of Bursa36
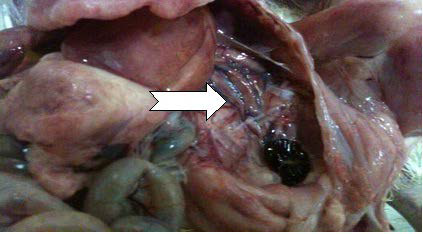
Histopathological Lesions
According to OIE,16 tissue of bursa of fabricius was removed aseptically from affected chickens in the early stages of the disease. Bursae is Chopped by using two scalpels, add a small amount of peptone broth containing penicillin and streptomycin (1000 µg/ml each), and homogenise in a tissue blender. The homogenate is centrifuged at 3000 g for 10 minutes. Harvest the supernatant fluid for use in the investigations. The bursal tissue are fixed by immersion in 10% buffered formalin and processed for histologic examination by standard methods of paraffin embedding, sectioning and haematoxylin and eosin staining.37 The tissue sections were examined by light microscope and the severity of lesions was graded on the basis of the extent of lymphocyte necrosis, follicular depletion and atrophy.38
Histological diagnosis is based on the detection of modifications occurring in the bursa. The ability to cause histological lesions in the non-bursal lymphoid organs, such as the thymus,10 the spleen or bone marrow39 has been reported as a potential characteristic of hypervirulent IBDV strains.
Necrosis and infiltration of heterophils and plasma cells occur within the follicle, as well as, the interfollicular connective tissue. In addition, a fibroplasia, the inter follicular connective tissue, may appear and the surface epithelium of the bursa becomes involutes and abnormal.40 Histological lesions in the kidney are nonspecific and probably occur because of severe dehydration of affected chickens. Lesions observed consisted of large casts of homogeneous material infiltrated with heterophils, and also glomerular hypercellularity.11 Proliferation of the bursal epithelial layer generates a glandular structure of columnar epithelial cells that contains globules of mucin. During this stage of the infection, scattered foci of repopulating lymphocytes were observed; however, these did not develop into healthy follicles.41
Virological Diagnosis
In the acute phase of infection, Infectious bursal disease virus may be detected in the bursa of Fabricius of chicks ideally within the first three days following the appearance of clinical signs.34
Virus Isolation in Cell Culture
In order to observe cytopathic effect, 0.5 ml of sample is inoculated into each of four freshly confluent chicken embryo fibroblast (CEF) cultures (from a specific pathogen free (SPF) source) in 25 cm2 flasks. Adsorb at 37 °C for 30-60 minutes, wash twice with Earle’s balanced salt solution and add maintenance medium to each flask. Then it is Incubated at 37 °C, observing daily for evidence of cytopathic effect (CPE). This is characterized by small round refractive cells. If no CPE is observed after 6 days, discard the medium, then freeze-thaw the cultures and inoculate the resulting lysate into fresh cultures. The more pathogenic IBDV strains usually cannot be adapted to grow in CEF unless the virus has first been submitted to extensive serial passage in embryos.16
Cell cultures containing 50% bursal lymphocytes and 50% CEF have been used to isolate and serotype IBD virus successfully.29 The traditional isolation method for IBDV using the chorioallantoic membrane of 9 to 11-day-old chicken embryos is no longer reliable, as some variant strains of the virus cause no embryo mortality.42 In addition, most field isolates cannot be readily adapted to grow in primary chicken cell cultures.43,44 However, embryo- or cell-culture adapted strains of IBDV replicate and produce cytopathic CPE in the avian continuous cell line45 and in mammalian continuous cell lines such as rabbit RK-13 cells46 and monkey Vero cells.47 Such cell lines provide sensitive media for assay of the virus.48,49
Virus Isolation in Embryos
To identify serotype 1 and serotype 2 inoculate 0.2 ml of sample into the yolk sac of five 6- to 8-day-old specific antibody negative (SAN) chicken embryos and on to the chorioallantoic membrane of five 9 to 11 day-old SAN chicken embryos. Specific antibody negative embryos are derived from flocks shown to be serologically negative to IBDV. Candle daily and discard dead embryos up to 48 hours post-inoculation. Embryos that die after this time are examined for lesions. Serotype 1 IBD produces dwarfing of the embryo, subcutaneous oedema, congestion and subcutaneous or intracranial haemorrhages but Serotype 2 IBDV does not induce subcutaneous oedema or haemorrhages in the infected embryos, but embryos are of a smaller size with a pale yellowish discolouration.16
Viral isolation was performed on bursas from infected chickens. Bursal tissue homogenate was inoculated onto the chorioallantoic membrane (CAM) of 10-day-old SPF embryonating chicken eggs.30
Serological Diagnosis
For serological investigations, usually blood can collected collect from the wing vein, allowed to clot and serum separated by centrifugation and stored at -20 °C until tested.50 Serological tests generally used for the detection of IBDV are ELISA, VN and Agar Gel Immunodiffusion (AGID). The ELISA is the most commonly used test for the detection of antibodies to IBDV.29 The infection usually spreads rapidly within a flock of birds. Because of this, only a small percentage of the flock needs to be tested to detect the presence of antibodies. If positive reactions are found in unvaccinated birds then the whole flock must be regarded as infected.51
Agar Gel Immunodiffusion (AGID)
Agar gel immunodiffusion test is one of the alternative tests recommended for IBD diagnosis by Organisation for Animal Health (OIE) in its list of tests for international trade.52 The AGID test is the most useful of the serological tests for the detection of specific antibodies in sera, or for detecting viral antigen or antibodies in bursal tissue. Blood samples should be taken early in the course of the disease, and repeat samples should be taken 3 weeks later. Because the virus spreads rapidly, only a small proportion of the flock needs to be sampled. Usually 20 blood samples are enough. For detection of antigen in the bursa of Fabricius, the bursae should be removed aseptically from about ten chickens at the acute stage of infection. The bursae are minced using two scalpels in a scissor movement, and then small pieces are placed in the wells of the AGID plate against known positive serum. Freeze-thaw cycles of the minced tissue may improve the release of IBDV antigens from the infected bursal tissue.51
The result of tested serum is interpreted as when a clear precipitin line is formed a “line of identity” with that of the positive control antiserum and with the antigen in the central well but, when no line is formed the tested sera is considered as negative result. The test is repeated when no clear precipitin line is formed at the positive control well or when a suspicious reaction at the tested serum well observed.53
AGID is the simplest, but least sensitive technique. Results are obtained after an incubation period of 48 h. Variability in results may be due to the investigator, as well as the nature of the viral strain used as an antigen.54,55,56,57,58
The AGID test can also be used to measure antibody levels by using dilutions of serum in the test wells and taking the titre as the highest dilution to produce a precipitin line.59 This can be useful for measuring maternal or vaccinal antibodies and for deciding on the best time for vaccination; however, this AGID quantitative determination has now been largely replaced by the ELISA.60
Enzyme Linked Immunosorbent Assay (ELISA)
The principle of ELISA is that antibodies are attached to their specific antigen by linking an enzyme to an antibody following the addition of the substrate. A serum sample is added and if there are specific antibodies they will bind to the antigen. If there is a positive sample, the antibody will attach and react with the substrate.61 Thus the positive samples will develop colour.62,63
The ELISA allows the quantification of antibodies to IBDV and is therefore used for monitoring the immune status of the chicken flocks to check response to vaccination, natural field exposure and decay of maternal antibody titer.64 The ELISA is the most rapid and sensitive method and presents the fewest variations due to the viral strain used as an antigen. It is economical, simple, and quick and tests a large number of samples at the same time and is adaptive to automation to computer software.64 However, ELISA cannot differentiate between the antibodies specific to the two serotypes.64,65
Agar Gel Precipitation Test (AGPT)
Another method used to detect antibodies to IBDV is the AGP test. This test has been adapted to the quantitative format.59 Antigen was prepared from a saline suspension of bursae from chickens infected with IBD virus. Briefly, a 50% suspension was homogenized and then clarified by centrifugation. The antigen was checked for sensitivity and specificity against known positive and negative sera but was not standardized otherwise. Test sera were placed in wells adjacent to positive control sera to enhance sensitivity and to establish specificity of lines.54
It is rapid but insensitive. It does not detect serotypic differences and measures primarily group-specific soluble antigens.64
Virus Neutralization Test (VNT)
In virus neutralization neutralization test (VNT) is the gold test and the only serologic test that discriminates between antibodies elicited by the two serotypes and various subtypes of the serotype 1 strains.66
VN tests are carried out in cell culture. The test is more laborious and expensive than the AGID test, but is more sensitive for detecting antibody. This sensitivity is not required for routine diagnostic purposes, but may be useful for evaluating vaccine responses or for differentiating between IBDV 1 and 2 serotypes.51
When a virus is mixed with homologous antiserum it will be neutralized and not infectious. This is the principle of a VNT and can be visualized in cell cultures. If the virus usually produces a CPE in cell culture, the neutralized virus will not be able to produce it and thereby the effect of the serum can be observed.61
| Table 1. Bursal Lesion Scoring System |
| Level of severity description |
| 0 |
No lesion |
| 1 |
Mild scattered cell depletion in a few follicles |
| 2 |
Moderate,1/3 to 1/2 of the follicles have atrophy or depletion of cells |
| 3 |
Diffuse, atrophy of all follicles |
| 4 |
Acute, inflammation and acute necrosis typical of IBD |
| Source6,7 |
Molecular Diagnosis of IBD
Another method that is used to detect IBDV is molecular technique. Reverse-Transcription Polymerase Chain Reaction (RTPCR) is one of the most important frequently used molecular method that is used to detect the genome of IBDV.68 Reverse transcription-polymerase chain reaction (RT-PCR) enable us to detect viral RNA in homogenates of infected organs or embryos, as well as in cell cultures, without considering the viability of the virus present.34 It is also used to detect the genome of viruses that don’t replicate in cell culture because it doesn’t require the growth of the virus before amplification. There are three steps in which RT-PCR is performed .These are; extraction of nucleic acids from studied sample, change of IBDV RNA into cDNA by Reverse Transcription (RT) and amplification of cDNA by PCR. The IBDV double stranded RNA stranded RNA (dsRNA) can’t be degraded by RNAases, unlike single stranded RNA.69
Extraction of IBDV RNA
Infectious bursal disease RNA can be extracted from infected tissues by using some kits which is available from commercial suppliers of molecular biology reagent. In another way IBDV RNA can be extracted by adding 1% sodium dodecyl sulphate and 1 gm/ml proteinase K to 700 µl of bursal homogenate and Incubated for 60 minutes at 37 °C. Nucleic acids are harvested from the final aqueous phase by ethanol precipitation and are resuspended in RNasefree distilled water or a suitable buffer. Water-diluted RNA should be kept frozen at a temperature below -20 °C until use.69
First Strand cDNA Synthesis
The extracted RNA is used for the synthesis of cDNA. The following reagents are mixed in PCR tubes to a final volume of 25 µL. These are; Template RNA 1 µg, OligodT primer 1 µL and Nuclease free water to 12 µL. Then the above mixture was kept at 65 °C for 5 min in a thermal cycler, followed by the addition of the following components in the indicated order: 5 X reaction buffer 4 µL, Rnase inhibitor 1 µL, 10 mM dNTP 2 µL and Reverse transcriptase 1µL. The above mixture was kept at 42 °C for one hour and 5 minutes at 70 °C in a thermal cycler.70 Synthesized cDNA was used as template for polymerase chain reaction (PCR).71
Reverse Transcriptase -Polymerase Chain Reaction (RT-PCR)
The cDNA was amplified in a 25 ml reaction mixture as given as below; Mastermix 12.5 µL, Forward primer (20pmol) 5’-GGTAACYGTCCTCAGCTTA-3’ 1 µL, Reverse primer (20 pmol) 5’-GTTCAGGATTTGGGATCAGC-3’ 1 µL, Template DNA 3 µL and Nuclease-free water 7.5 µL. After amplified the reaction mixture was subjected to initial denaturation of 95 °C for 5 minutes followed by 35 amplification cycles at 95 °C for 45 seconds, 51°C for 45 seconds and 72 °C for 1.30 minutes with final extension at 72 °C for 10 minutes.70
The extracted RNA is changed into cDNA then RTPCR. Reverse Transcription-Polymerase chain reaction is work on VP2. The VP2 is the major structural protein that contains the antigenic regions responsible for the production of neutralizing antibodies in the chicken. VP2 contains a hypervariable region that displays the greatest amount of amino acid sequence variation between strains.72,73 This region is responsible for antigenic variation, tissue-culture adaptation and it is partially responsible for viral virulence.74
A commercial cDNA synthesis kit (Fermantas, USA) is used to make cDNA. Hair-Bejo M4 also reported that for amplification of a 743 bp fragment of VP2 hypervariable region, two region, two primers are used these are; forward primer 5’-GCCCAGAGTCTACACCAT-3 and Reverse primer s5’-CCCGGATTATGTCTTTGA-3’. Then the amplification products were detected by gel electrophoresis in 1.5% agarose gel in buffer. Gels were run for 1.5 h at 80 V, stained with ethidium bromide (0.5 µg/ml), exposed to ultraviolet light and photographed (Visi-DocIt system, UVP, UK).
If four amino acids (alanine 222, isoleucine 256, isoleucine 294 and serine 299) are present simultaneously present, it simultaneously, it is considered as indicative of vvIBDV.75,76
CONCLUSION AND RECCOMMENDATIONS
Infectious bursal disease is one of the viral diseases that affect poultry all over the world. It mainly affects young chickens between 3-6 weeks old. This disease is highly affects bursa of Fabricius. Economic losses due to IBD is directly associated with mortality and in directly with immunosuppression. Infectious bursal disease is also called “gumboro disease” according to the location of first outbreaks in Gumboro Delaware, USA, was initially described avian nephrosis due to damage seen in kidney but later the name of avian nephrosis is changed to infectious bursal disease depending on morphologic and histologic change occurred in bursa of Fabricius. Infectious bursal disease virus is the highly contagious disease that causes IBD. Two serotypes are recognized. These are; serotype 1 which is pathogenic to chickens and serotype 2 which are not pathogenic to chickens. Diagnosis of IBD is depending on clinical signs, differential diagnosis, gross lesions, histopathological lesions, virus isolation, serological and molecular diagnosis. Agar gel immunodiffusion is the simplest but least sensitive where as ELISA is a rapid and sensitive method a rapid and sensitive method is but cannot differentiate serotypes. Virus neutralization test is the golden standard and the only serologic test that differentiate test that differentiates antibodies of two serotypes and sensitive but sensitive but it is more laborites laborious and expensive than AGID. Another method that is used to detect IBDV is molecular methods. Reverse Transcription-Polymerase Chain Reaction is used to detect IBDV without considering the viability of the virus by working on VP2 which is a major structural protein that is responsible for the production of neutralizing antibodies in chickens and it is a place where a greatest amount of amino acid variation occurred between strains. Depending on the above conclusion, the following recommendation is forwarded.
Early diagnosis of IBDV must be conducted because of it is highly contagious disease. Infectious bursal disease should be differentiated from other disease that has the same lesions. More sensitive and rapid test should be selected.
ACKNOWLEDGEMENTS
First, I would like to thank the almighty Allah, for giving me health, peace, love, energy and suitable condition throughout my life. Next I would like to passionately express my thankfulness to my advisors, Dr. Tesfaheywet Zeryehun.
Also, I wish to convey my sincere thanks to HUCVM library, computer and internet center staff for their kind operation.







