INTRODUCTION
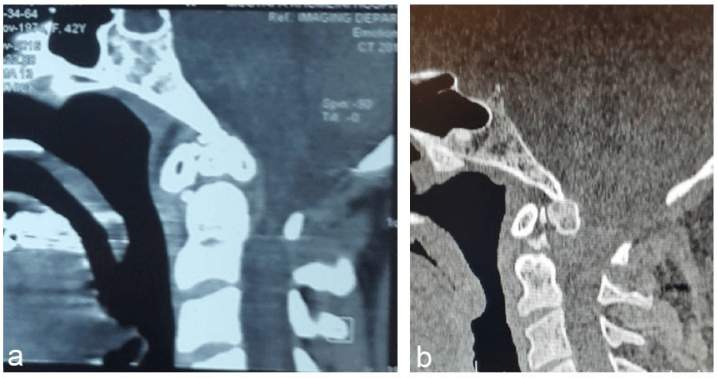
Os odontoideum (OO) or separate odontoid is a well-known pathology that was described for the first time in the 19th century. The word OO has a Latin origin derived from os (bone) and odontoideum (tooth-like). There is an existing controversy as to whether the etiology of OO is characterised as a congenital or traumatic condition. In 1980, Fielding et al1 classified OO into 2 anatomic types, dystopic and orthotopic, on the basis of the relationship of the separated odontoid ossicle to the axis and the clivus. In the orthotopic type of the condition, os is in its normal position and it maintains a normal relationship with the clivus, atlas and the axis (Figure 1), thus, available for free movement with the anterior arch of the atlas. However, a dystopic os lies close to the base of the clivus or is fused to the basion Figure 2 thus, available for movement with the clivus.
Figure 1: (a, b, and c), Three Different Cases with os Odontoideum of Othotopic Type
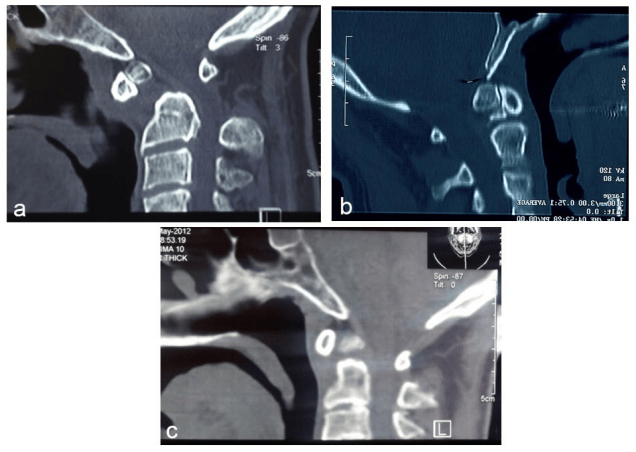
Figure 2: (a and b), Two Different Cases with OS Odontoideum of Dystopic Type
On the basis of an increasing risk of cervicomedullary compression, OO has been classified as asymptomatic or symptomatic conditions. A minority of the patients with OO might continue to be asymptomatic throughout their life and may be diagnosed incidentally. In the case of patients with symptomatic OO, the clinical manifestation of this pathological condition may be characterised by neck pain, progressive quadriparesis due to atlanto-axial instability, and vascular insults secondary to vertebral artery kinking or thrombosis elucidating 3 distinct clinical symptoms of OO, which might occur in isolation or in association with one other. A dystopic OO is generally more prone to cervico medullary compression relative to an orthotopic OO. This pathological condition is mostly diagnosed among children and young adults, although its discovery in the middle age group is not infrequent.1,2,3,4,5,6,7 This condition might be rarely diagnosed in the elderly, either incidentally or on account of a neurological deficit.8
The ability to manage the possible cases of OO is determined by the stability of atlantoaxial joint.8,9 While OO under stable conditions might be considered for close observation and periodic radiological assessment, prophylactic stabilization might be considered for examining asymptomatic conditions with implied instability.5,9 Thus, instability, which characterizes the asymptomatic condition of OO, may put the patient at a risk of developing significant spinal cord injury even when subjected to minor trauma.1,2,4,5,6,7,10 However, the cardinal cause underlying the signs and symptoms of a patient with neurological deficits is attributed to atlantoaxial instability, which demands surgical intervention. Before surgery, however, the reducibility of atlantoaxial dislocation should be determined.
In symptomatic patients with reducible atlantoaxial dislocation (RAAD), the primary strategy of treatment is posterior atlantoaxial fixation with the application of an available osteosynthesis method and arthrodesis.11,12,13 In the current scenario, the surgery of RAAD has been proven to be safe enough to be under taken to address any possibility of the presence of instability.
In irreducible atlantoaxial dislocation (IAAD), the most reasonable line of treatment is the protection of the cervico medullary region from further compressive forces. However, the most challenging aspect of performing the surgery will be to relieve the cord compression, which can be achieved either by reducing the deformity or by mediating the removal of the pathology underlying this clinical condition.3,11,14-25 This indicates that two different strategies can be implemented for the management of IAAD: (a) a resection strategy confined to the removal of the anomaly via different approaches and (b) a releasing strategy confined to the anterior or posterior release of C1-C2 facet joints. Regardless of the strategy implemented, the procedure should be followed by a step of internal fixation. A thorough review of the history, embryology, pathogenesis, clinical and imaging features, as well as the mode of treatment in OO has been discussed in the following paragraphs.
DISCUSSION
Definition: The hypoplasia of the odontoid process associated with the oval ossicle, widely separated from the body of the axis, is termed as OO.
History: The early description of OO dates back to the third quarter of the 19th century, where detailed anatomical and autopsy studies of this pathological condition were undertaken by Bevan in 1863, Giacomini in 1886, and later by Cunningham in the same year.26,27 In the 20th century, this clinical condition was discussed by Le double in 1905 and later by Mixter and Osgood in 1910.26,28
The significance of the clinical and radiological aspects of OO has been described by Scinz in 1923 and subsequently by Lombard in 1945 and Ford in 1952. In 1953, McRae described the radiological features of separate odontoid in 17 patients including 2 children.29,30 Later, several papers in English Literature were reported describing a single case or small to large series representing this clinical condition.26-66
Embryology: The embryology, growth, and development of the odontoid process is complex. The centrum of the fourth occipital sclerotome (proatlas) forms the apical cap of the dens or ossiculum terminalis. The remaining portion of the odontoid process or most of the architecture of the odontoid process originates from the mesenchyme of the sclerotome of the first cervical vertebrae. In the early stages of development, the centrum separates from the provertebra of C1, moves caudally, and then joins the axis to become the odontoid process. Later, when the first spinal sclerotome or ossiculum terminalis undergoes fusion, the formation of the rest of the dens is complete. Failure to fuse results in the development of a small ossicle at the tip of the odontoid process known as ossiculum terminal, which is rarely of clinical significance. The fusion of the two centers of ossifications into a single mass occurs during the period between the fifth and sixth prenatal months, but it becomes radiologically visible at around the age of 3 years and completely fuses with the body of the dens by the age of 12 years.
The second spinal sclerotome (C2) contributes to the caudal part of the axis body and arches. There are three other primary ossification centers in the axis besides the 2 centers for the odontoid process on either side of the midline, one for the body and two for the neural arches.67
At birth, the dens are separated from the body of C2 by a wide cartilaginous plate. This plate, the dentocentral synchondrosis, lies below the anatomic base of the dens, within the body axis. The process by which the dens reunites to the body, begins in the 4th year of life, and fusion by synchondrosis is thus, completed having attained a certain age, although it might be delayed until age of 12 years.7
Pathogenesis: Since the beginning of the discussion on OO, its etiology has been a debatable issue. The debate concerning the etiology of OO can lead to controversies underlying the medicolegal setting and consequently, result in inappropriate treatment. In 1953, McRae explained the embryological origin of OO.30,68 According to the study, this pathological condition is not implicated in clinical symptoms until the ongoing traumatic condition evokes atlantoaxial instability and neurological deficits. This was supported by several authors, including Davis and Gutierrez who reported 4 cases, indicating a congenital etiology for this clinical condition.69 Later, Currarino proposed that OO represents an abnormal condition, on account of an incomplete or partial embryonic segmentation of the mid-portion of the odontoid between the chondrification segments, X and Y.70
The familial forms of OO have been observed in identical twins and siblings with no existing historical evidence of trauma in support of the congenital hypothesis.63,71,72 Supporting the evidence of the congenital origin of OO, it has been widely observed among patients with congenital anomalies. The coexistence of OO with aplasia of the anterior arch of the atlas, split atlas, and bipartite atlas, the absence of the posterior arch of the atlas, the hypertrophy of the posterior arch of the atlas, and Klippel Feil syndrome is indicative of its congenital origin.73 In 2011, Yang et al reported the case study of a 34-year-old female with unilateral cleft of the posterior arch of the atlas in association with OO.74 Among other evidences supporting the congenital origin of OO, might be the observations recorded from cephalometric radiographs through experimental studies, undertaken for the evaluation of dental anomalies in children.75,76,77
However, this theory has been questioned on account of the location of the neurocentral synchondrosis below the level of the superior articulating facet, whereas the gap in OO is frequently located above the plane of the superior articulating facet.5,10 The implication of OO in individuals suffering from Down syndrome and skeletal dysplasia, such as Morquio disease, multiple epiphyseal dysplasia, achondroplasia, pseudoachondroplasia, Larsen syndrome, and chondrodystrophia calcificans congenital are in favour of its congenital etiology.9,72,78,79 The association between OO and achondroplasia was first reported by Gulati and Rout in 1974, and since then, 5 more children with related symptoms have been reported.9 However, this coexistence in achondroplastic adults is an extremely rare occurrence and has been reported in the intensive research undertaken by Rahimizadeh et al in 2015.9
In 1999, Taggard et al reported the study of 33 patients with Down syndrome who had craniocervical instability. Of these 33 patients, nearly 14 of them were reported to have experienced atlantoaxial instability, such that OO has been identified as the cause of instability for nearly 10 of them.14 In 2005, Nader-Sepahi reported the case of 10 children with Down syndrome who experienced atlantoaxial dislocation due to OO.58,80
On the contrary, there are recorded cases of patients who have experienced injury in the odontoid process during infancy or childhood, thus causing avascular necrosis of its tip, such that its physiological representation is similar to that of OO.
The post-traumatic theory defining the origin of OO stems largely from the work of Fielding et al, who hypothesized that an unrecognized fracture of the dens followed by the contraction of the alar ligaments, leads to the upward dislocation of the fragment from its normal site. The position of the fragment adjacent to the ring of the atlas is maintained by the transverse ligament. Because of the disruption in the blood supply to the base of the odontoid under traumatic conditions, the nutritive requirement of the distracted ossicle is addressed by the proximal arterial arcade. This condition eventually leads to the development of OO.1 According to pre-existing studies, 17 out of 35 patients with OO were recorded to have a history of cervical spine trauma of sufficient intensity.1 In 1999, Menezes while examining 260 patients with OO found that early childhood trauma to the craniovertebral junction (CVJ) had been recorded in 74 patients, of which 30 patients with normal odontoid processes were documented following the initial examination before the patient reached an age of 4 years.3 In addition, several authors cited the location of the separation and longitudinal studies documenting the eventual posttraumatic development of OO in patients whose cervical spine radiographs were initially normal after the injury.81-83
One of the examples is that of Schuler et al82 report in 1991, which documented the development of OO after a significant period of trauma. This study reported the natural history of the abnormality observed in a 2-year-old girl who experienced neck pain after a significant fall. Cervical radiographs obtained immediately after the traumatic incident, within a span of 1 and 3 months following injury, were not of great significance. Following four months of injury, the resorption of the dens became evident, and after a span of 13 months, the typical appearance of an OO was demonstrated on plain films.
Critics of the congenital theory commented that older patients often could not recall injuries sustained in their childhood and may thus underreport pre-existing trauma. For example, in 2004, Fagan et al Reported 18 cases where the history of a potential traumatic etiology was only obtained in 1 of the 18 (6%) reported cases in their series.65
As an opponent, Sankar et al recently reviewed the medical records and imaging files of 16 reported cases of OO. Only eight of the 16 patients reported a previous history of trauma and only 3 of these 8 injuries occurred with an interval, remote enough to allow the remodelling of the dens to an ossicle by the time of presentation. On the basis of the recorded data, there are two possible etiologies underlying OO: posttraumatic and congenital.59 However, we acknowledge the availability of sufficient data and literary records in support of the possible etiologies associated with OO.
Age and gender: OO is more prevalent among males and is usually seen in the second and third decades of life. It is uncommon in middle age and is rare in the elderly. In 2011, Rahimizadeh and Habibi reported the case of a 67-year-old male with OO complications complemented with myelopathy and reviewed the previously reported cases in this age group. These studies indicated that following the first instance of OO with myelopathy reported by Wollin in 1963, 9 more cases have been published.9,40,84
Clinical picture: Although OO has a clear definition, its clinical manifestations are variable among patients defined by the spectrum of severity of symptoms ranging across the categories of being completely asymptomatic or with neck pain to severe cervicomedullary myelopathy. In fact, patients with OO might remain asymptomatic throughout their life or become symptomatic as early as in childhood. Therefore, with consideration of these two distinct clinical parameters, it was rational to classify the subjects with OO into two categories of patients: symptomatic and asymptomatic.
Asymptomatic Patients
This group represents subjects with OO who might continue to be asymptomatic for a long period of time or throughout their life, such that its clinical pathology was discovered incidentally by imaging done for other purposes.77,85 In 1989, Tetradis and Kantor reported its discovery in cephlometry for the evaluation of dental anomalies of the children laying down the foundations for further research in this domain.75,76 The description of the natural history of OO is very limited and is confined to a few case reports describing the clinical symptoms of patients who were treated with great attention without any recorded accident. Eight out of the 37 cases reported by Fielding et al, were that of an asymptomatic clinical condition of OO such that all the 8 subjects continued to remain asymptomatic during the follow-up.1,86 Similarly, all five of the 15 asymptomatic patients who were treated with great attention, as reported by Dai et al, continued to show no symptoms of the disease.4 On the contrary, Dyde believed that all of his patients with OO sooner or later developed cord compression.38 This was supported by the observation that only 2 of the 17 reported cases of OO described by Minderhood and Braakman did not result in neurological deficits in the follow-up.46 The natural course of OO toward instability was also supported by Clements et al in 1995, who reported the case of a patient with documented stable OO. The patient developed an atlantoaxial dislocation during the course of a 5-year follow-up.87 In one of the largest series of studies, reported by Klimo et al, up to 15% of the 78 subjects with OO were asymptomatic either with stability or minor instability. However, these authors believe that, regardless of the radiographic feature of stability, many of these seemingly stable OO are actually unstable, quite similar to odontoid type 2 fracture where the atlantoaxial joint is supported only by ligamentous structures.5,10
Prior research indicates that patients with the asymptomatic condition have been doing well over a number of years without proper treatment.7 At the same time, cases of sudden spinal cord injury in patients with OO after minor trauma have been reported.3 The clinical manifestation of this disease is implicated following any minor or trivial traumatic condition as mild as a neck pain. This condition might be diagnosed only in autopsy if sudden death results following the period of trauma.
The first incidence of this condition was reported by Symonds et al in 1937, when a 47-year-old man with an asymptomatic separate odontoid became quadriplegic and ultimately died following a car accident.88 Later in 1969, Michael et al reported the death of a 35-year-old taxi driver who underwent a minor car accident. On the basis of the autopsy reports, a separate odontoid was found.89 Other examples illustrated in the works of Minderhood and Braakman, reported 4 of 13 cases in which the patients developed severe neurological deficit when subjected to different traumatic conditions. Before any event of trauma, all the four reported cases were asymptomatic.46 Kim et al in 2011 described how a minor trauma can provoke neurological deficit in an asymptomatic patient.90 This means that a lack of instability at the atlantoaxial joint as observed in the initial radiographs does not guarantee that the condition will permanently remain stable and instability will not necessarily develop over time. In 1989, McGoldrick and Marx reported the case of an asymptomatic 33-year-old man with OO who sustained central cord syndrome following a car collision.91 In 1990, Dempster and Heap reported the case of a patient with asymptomatic OO who died because of fatal cervicomedullary injury in a car accident.92
Acute neurological deterioration after trauma has also been reported among 63 patients out of 134 reported cases with OO as reported by Menezes.3,78 In 2010, Zhang et al reported a study of the fatal traffic accidents undergone by patients with asymptomatic OO among whom, 6 children with OO sustained major trauma. Of the 6 reported cases, 2 patients were asymptomatic before trauma. Central cord syndrome appeared in one case and Brown-Sequard syndrome in the other when subjected to minor trauma.93 A review of these studies may suggest that only a small number of patients with incidental OO can be treated effectively with appropriate treatment, but there is less than definitive evidence regarding the natural history and risks associated with untreated OO.10
Nonetheless, close clinical observation and periodic radiological investigation are necessary for the examination of asymptomatic patients with stable OO who might continue to retain this condition for many years, but may become myelopathic or experience sudden death following a minor trauma.10,87,90,91
Symptomatic Patients
The clinical features of OO were first discussed by McRae in 1953.30 Later, Rowland et al classified the clinical perspective of this pathological condition into four groups.94,95 However, according to the literary analysis of recently published research, a patient with OO usually experiences 3 clinical symptoms manifested as suboccipital or cervical pain, cervico medullary myelopathy, and vascular insufficiency.
Cervical and suboccipital pain is usually the initial symptom of OO followed sooner or later by a wide spectrum of characterizing attributes such as cervicomedullary compressive myelopathy ranging across the milder symptoms such as Lhermitte’s sign to quadriparesis with hyperactive reflexes, Hoffman’s sign, which could lead to severe debilitating conditions, and upward-going toes or Babinski’s sign. However, when OO is underdiagnosed, chronic atlantoaxial instability with cervico medullary compression could consequentially lead to a progressive neurological deficit.33 This indicates that the signs and symptoms associated with the atlantoaxial instability implicated in OO, are similar to the instability attributed to any other cause, which could possibly be traumatic, neoplastic, or inflammatory.
There is often a history of trivial, significant remote or recent trauma, which often makes it difficult to distinguish between an odontoid fracture and a congenitally separated odontoid. The instability observed in the spinal cord at the cervico medullary region in response to trauma, might be exacerbated by the formation of a retro-odontoid pseudotumor and retro-odontoid synovial cyst.
It is noteworthy to pay attention to the possibility of retro-odontoid pseudotumor formation and growth in OO, which is further complicated by atlantoaxial instability. Therefore, it has been hypothesized that a chronic atlantoaxial instability is the predisposing factor leading to a retro-odontoid pannus.73,96,97,98
Retro-odontoid pseudotumor, also named pannus, is a non-tumoral, fibrocartilaginous, or granulation tissue that can compress the already compromised spinal cord at 12 cervicomedullary junctions. Surprisingly, the pannus usually resolves and disappears after atlantoaxial fixation, indicating that instability is the cardinal cause of its formation.
Rarely, signs of the vertebral artery insufficiency may be encountered and complicate an OO varying from simple vertigo to massive cerebellopontine infarction. The effect on the vasculature is an impact of the kinking of the vertebral artery at foramen transversium. In 1952, Ford reported the case of a patient with OO who was affected by syncope and vertigo, attributed to the intermittent obstruction of the vertebral artery.29 In 1991, Bhatnagar et al reported the first incidence of cerebellar infarction arising due to OO.99 Two more cases were reported in 1994 and 2003 by Miyata et al and Fukuda et al, respectively.100,101 Takakuwa et al reported intermittent vertebral artery kinking due to OO in 2 out of 7 patients with craniovertebral anomalies, who suffered from posterior circulation attacks due to asymptomatic OO.99,101,102,103
In addition to the above mentioned clinical symptoms, sleep apnea rarely is observed among patients suffering from OO with an anterior atlantoaxial subluxation. In other related cases reported, sleep apnea results on account of the narrowing of the airways due to subluxation. Under very rare circumstances, obstructive sleep apnea is associated with a central component. Central sleep apnea results due to a cervico medullary myelopathy at the level of C1 and the extension of edema to the lower medulla. These areas are key sensors for elevated levels of CO2, such that any damage incurred can result in a respiratory drive. Central hypoventilation or Ondine’s curse characterized with periodic sleep apnea has been rarely reported in patients with OO.104,105
Imaging
Two different types of OO have been defined based on the position of the dens tip: orthotopic and dystopic. In the orthotopic type, the dens is in an anatomical position. In dystopic OO, the ossicle may fuse with the clivus at the basion.106 Most of the reported cases of OO are of the orthotopic type. In only 22 out of the 134 cases reported by Menezes, was the mention of the fusion of the os to the clivus. An exception to the cases reported were the case series recorded by Ding et al such that 8 of the 12 reported cases were of the dysplastic type.107 OO is associated with a rounded, smooth cortical border that was separated by a variable gap from the remaining odontoid process. Plain X-rays and tomograms may observe OO with intact cortical margin above the axis.
On the basis of the study undertaken by Matsui et al in 1997, OO was classified into three types according to its configuration on anteroposterior open moth radiographs. There have been evidences of round, cone, and blunt tooth types of OO. They believe that the round type is commonly observed among females and myelopathy is more prevalent in this type.52 The relevance of this classification remains questionable.
Dynamic flexion and extension radiographs are quite informative for examining the instability in an OO.106 (Figure 1). In the orthotopic type, flexion-extension radiographs may demonstrate that the os moves independently and relative to the axis. The direction of atlantoaxial dislocation can be determined by dynamic X-ray analysis, in which an OO might move anteriorly or posteriorly or be free-floating, thereby showing free movement in both the directions (Figure 3). OO with posterior dislocation is less commonly observed among patients (Figure 4). Fielding et al reported the case of 22 patients (67%) with anterior instability, 5 patients (15%) with posterior instability (mean posterior translation of OO during an extension of 8.4 mm), and 8 patients (23%) with both anterior and posterior instability or show free movement in both the directions. Three patients (9%) were reported to have 3.0 mm of C1-C2 mild motion and 3 patients (9%) who showed no detectable C1-C2 motion.1 Plain radiographs are often sufficient for the diagnosis of this rare pathology. However, the degree of C1-C2 instability identified on the basis of cervical X-ray studies, does not correlate with the presence of myelopathy. Spierings and Braakman observed in their series of 37 patients that it was the smallest diameter of the spinal canal at C1-C2 that correlated most closely with permanent spinal cord injury and not the degree of instability. This data suggests that when the diameter of the spinal canal at C1-C2 level on sagittal view (also known as space available for cord or SAC) is less than 13 mm at the C1-C2 level, it correlates with myelopathy detected by clinical examination.
Figure 3: Free floating Os odontoideum: (a) Flexion, (b) Extension
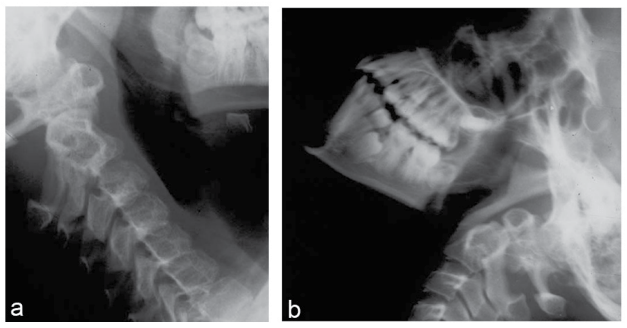
Figure 4: Posteriorly located Os Odontoideum
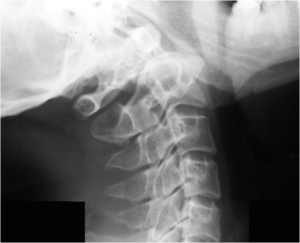
Some craniocervical anomalies such as occipitalization of the atlas, Klippel Feil syndrome, and basilar invagination are more commonly observed among patients with dysplastic OO.107 Verjaal and Harder demonstrated the absence of the anterior arch of the atlas in their study. According to the studies reported by Wollin, the posterior arch of the atlas might be bifid in OO.40 A sclerosis of the posterior arch was recorded by Schultz et al108 One of the cases reported by Gillman and Wadia respectively, observed the condition of Klippel Feil anomaly.34,41 Yang et al reported the introduction of an unilateral cleft of the posterior arch of the atlas in a 34-year-old female with OO.74
Furthermore, in acute cervical injuries, distinguishing OO from a dens fracture can be critical, as many characteristics of OO may overlap with a healed dens fracture. The smooth, well-corticated, sclerotic margins and round or oval shape as observed can help distinguish this pathological condition from non-corticated dens fractures with irregular margins of an acute type II odontoid fracture. However, a high-resolution multiplanar computed tomography (CT) scan might be necessary to observe the detailed anatomy of OO and any associated cervical or skull base anomalies for planning surgical action.
In 2004, Fagan et al measured the atlanto-dens joint interval in the mid-sagittal reconstructed CT. They observed a narrowing of the cartilage space at the arch-peg area and interdigitating of the two joint surfaces. This was named as a jigsaw sign and was believed that it is only observed among patients with OO having a congenital origin (Figure 4). 65 According to Fagan et al, a jigsaw sign is not observed in a non-union odontoid fracture with atlantoaxial dislocation.65 The hypertrophy of the C1 anterior arch described as a relevant symptom to distinguish OO from acute dens fracture is best observed in reformatted CT, as the hypoplasia of the atlas arch, biparate atlas, and its agenesis are other avenues worth exploring in defining the natural course of radiography in asymptomatic patients with OO, where the pathology is diagnosed incidentally. CT angiography for the examination of vertebral arteries, and its possible encroachment in the foramen transversium of a displaced atlas, might be necessary for surgical intervention. Overriding vertebral artery at the C2 level can be demonstrated through coronal reconstructed axis images, which is necessary if C2 pedicle screwing is foreseen.
Magnetic resonance imaging (MRI)
An MRI study should also be done to evaluate any intrinsic spinal cord signal changes known as myelopathy. (Figure 5) It is the modality of choice for the demonstration of myelopathic changes in the cord presenting itself as a hyperintense signal intensity lesion in T2-weighted MRI. This is usually demonstrated as a small intramedullary hypersignal lesion in T2-weighted images and as a hypointense lesion in T1-weighted images. Occasionally, T2 hyperintensity and contrast enhancement at the cervicomedullary junction might be extensive and hence mimic other pathologies such as intramedullary tumors (Figure 6). In 2011, Gigante et al reported the study of 2 patients whose initial clinical presentation and MRI findings were suggestive of an intramedullary neoplasm but whose ultimate diagnosis indicated cervical spine instability and cord injury due to OO.109 Syringomyelia or small syrinx might be seen with OO (Figure 7). These characteristic features attributed to OO appear as diminished or disappeared after C1-C2 fixation.
Figure 5: Small Hyperintensity at Cervicomedullary Junction
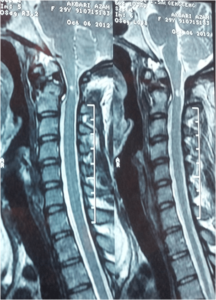
Figure 6: Large Hyperintensity Indicating Myelopathy
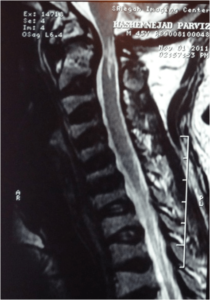
Figure 7: Hyperintense Pathology at Cervicomedullary Junction, Not a Syrinx at Mid-cervical Region
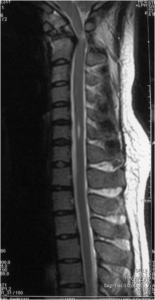
In 2003, Lee et al demonstrated the instance of both extensive T2 hyperintensity and contrast enhancement, which might occur at the site of maximal cord compression. These features are mostly resolved postoperatively. Rarely, a synovial cyst or retro-odontoid mass (pannus) may develop as a result of the abnormal motion at the vicinity of OO (Figure 8). MRI also indicates high sensitivity in revealing these pathologies.
Figure 8: Pannus Formation at the Site of OS Odontoideum
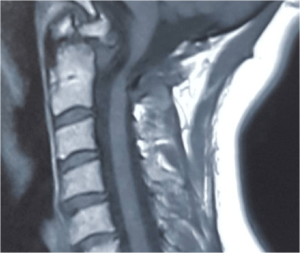
The concurrence of OO with cervical spondylosis or cervical spondylotic myelopathy can be best demonstrated and evaluated through the application of MRI; OO with instability is best examined by dynamic radiography, but the condition might be missed on MRI recordings, if it is used in the first diagnostic study.110
In MRI, a retro-odontoid synovial cyst shows uniform low intensity on T1-weighted images and uniform high signal intensity on T2-weighted images. Gd-DTPA-enhanced T1-weighted images usually show rim enhancement of the mass with no internal enhancement. Pseudotumor in OO with instability is usually observed as an isointense mass. Faint enhancement is demonstrated in Gd-T1-weighted MRI. Kinematic real-time MRI is a new imaging modality that can provide additional information about instability. By application of real-time MRI, subluxations that are not visible or conclusive in dynamic radiographs can be demonstrated.111
Treatment
Because of the rarity and variable clinical picture of OO, treatment modalities are inconsistent. It is important to understand that the strategies for the optimal treatment of OO have changed with time. Thereby, having reviewed the literature carefully, we have attempted to prepare an accepted guideline for the treatment of this pathological condition.
Conservative: Some authors believe that observations suffice in asymptomatic subjects with a stable spine. Some surgeons do not advise any treatment for radiographic stable OO in asymptomatic subjects.7,31,112 Many of these individuals may remain stable and free from neurological deficits in the follow-up. However, patients or their relatives should be informed adequately of the risk inherent in conservative management. Observations and periodic flexion-extension cervical radiographs are recommended to be recorded at an annual interval, as some of the stable conditions might result in instability with the passage of time.
Despite a few prophylactic surgeries reported in the literature, it should be emphasized that prophylactic surgery has not been supported widely in stable asymptomatic patients, except those associated with intense sports activities. These surgeons believe that the risk of operation may be higher than the risk of sudden instability and neurological impairment.7,31 In 1982, with respect to high mortality observed in surgery, Spierings and Braakman advocated conservative treatment in more than 60% of their cases, including among those who were asymptomatic. In their study, asymptomatic subjects did not show any characterizing symptoms during the follow-up evaluation.7 Furthermore, in a paper published by Dai et al, in 2000, 7 out of the 44 patients with OO were asymptomatic, but only 5 could be recorded.4 It was suggested that all the 5 subjects remained stable during the follow-up. Another example of this belief is reflected in a large series reported by Fielding et al in 1980. Eight out of the 35 patients with OO, but without radiographic signs of C1-C2 instability, were doing well with non-operative treatment.1 On the contrary, Klimo et al believed that although the cruciate ligament in the absence of the transverse ligament might be sufficient for routine daily activities, it apparently cannot resist stressful events and might easily lead to spinal cord injury.5 Accordingly, he described OO as an unstable condition and advocated surgical intervention, regardless of the clinical presentation.
Furthermore, some others believe that dynamic cervical X-ray is not enough to conclude about the stability of OO. These authors proposed kinematic MRI, which show instability more accurately, even in those flexion-extension radiographs which have already ruled out instability. It is well known that an asymptomatic stable OO might become unstable even after a minor trauma, resulting in rapid progressive neurological deficit and ultimately death.
Nonetheless, taking into account sudden death or significant neurological morbidity when subjected to minor trauma, this condition is debatable.91,92,93 Furthermore, as this condition is more commonly observed in children and young adults who are subject to high-energy traumas, parents should be informed about the possibility of sudden displacement of the dens due to traumas inherently associated with some sports. Therefore, the surgeon should emphasize on the necessity of periodic neurological examination and dynamic radiographs for examining such patients. Also, parents should monitor the activities of such children and advise them to avoid high-energy sports.
We believe that even in stable OO, the treatment should be integrated with careful examination on a case-by-case basis, particularly after analysing the activity level of affected children. Moreover, for noncompliant patients and for those with Down syndrome, prophylactic surgery was recommended.
Surgical treatment: The management of asymptomatic subjects with apparent atlantoaxial instability has thus, been proven and these subjects have been recommended surgical stabilization. Actually, the surgical management of asymptomatic instability has been justified because of the risk of severe atlantoaxial dislocation with permanent damage of the spinal cord and even death in response to relatively minor to moderate intensity traumas, as has been reported by most of the authors. The distinct morphology of the dens relative to the body axis, along with its counterpart atlas with respect to the shape, lack of intervening disc, and anterior synovial articulation, which are quite different from subaxial vertebra, was the subject of initial frustration with high failure, high morbidity, and even mortality for several decades.
This continued until the surgeons confronting OO became more familiar with the detailed anatomy of the complex, neighboring vital structures. By far, with the improvement of imaging tools, a greater number of patients with this pathology were recognized, and with the advancement in surgical techniques, the overall results of surgical management and outcome were maximized and some accepted guidelines were advocated. Gradually, the definite role of surgical intervention in symptomatic patients and asymptomatic unstable conditions was recognized. Furthermore, the questionable role of prophylactic surgery in asymptomatic stable situations was reviewed.
Klimo et al believed that patients with incidentally discovered OO and instability in dynamic cervical radiographs should be considered for surgery. He strongly recommended surgical stabilization for those with even mild instability and those who were greater than 20- years-old with respect to a well-developed anatomy, considered suitable for screw insertion. Some other surgeons believed that asymptomatic patients without apparent instability may be monitored with serial radiographs and clinical evaluations to detect whether instability or significant symptoms develop, in contrast to Klimo et al who advocated surgery even in this group of patients. Arvin et al recommended that patients with a condition of stable OO should be clinically monitored and evaluated with dynamic plain X-rays annually. Furthermore, he preferred to implement the performance of control MRI in a 5-year interval. According to both the studies, the avoidance of all contact sports is advocated.6,10
Management of Symptomatic Subjects
Symptomatic patients require surgical intervention. The surgical strategy in the management of OO is quite different in the case of reducible relative to irreducible atlantoaxial instability. Therefore, for a better understanding of the surgical management, the classification of the instability as reducible and irreducible is mandatory. This classification dates back to the reported studies by Greenberg et al in 1968, when he classified atlantoaxial dislocation as reducible (RAAD) or irreducible (IAAD).
Management of Reducible Atlantoaxial Instability in OO
In RAAD, the mainstay of surgery is aimed towards C1-C2 fixation. For this purpose, a variety of techniques have been proposed. Historically, posterior C1-C2 fixation has been considered as a promising topic for research since the beginning of the 20th century. Initially, a majority of these techniques could result in limited stabilization and were actually associated with semirigid fixation. By far, these procedures have been initiated by performing C1 arch-C2 spinous process stabilization which were implemented by passing a strip of fascia lata under the lamina of C1 arch and by knotting it around the C2 spinous process with interrupted silk sutures. Subsequently, Mixter and Osgood modified this technique by passing a braided silk soaked in compound tincture of benzoin under the posterior arch of the atlas and tying it about the hooked spinous process of the axis.28 Later, Willard switched to the use of wire for C1-C2 fixation and subsequently its augmentation with iliac bone graft. Subsequently, Gallie modified this technique and described its thorough detail in 1939, which contributed to its popularity thereafter. Since then, several different and modified techniques of wiring have been described by other surgeons.49,113,114
In 2002, Brockmeyer used cable instead of wire for C1-C2 stabilization. In all these techniques, mechanical stability is achieved with structural tricortical iliac bone blocks fixed in compression between C1 and C2 with either wire or cable.115 For a long period of time, many patients with radiographic signs of instability were only treated using these techniques. For example, in the case series reported by Sankar et al in 2006, 15 out of the 16 reported cases underwent posterior wiring fixation and fusion where, in only one case, instability was managed with transarticular C1-C2 instrumentation.59 Out of the 13 cases of OO reported by Behari et al, the modified Brooks technique was used in 10 cases.116 However, it should be noted that these techniques are not effective against rotatory forces and this drawback may be a major cause of its failure.117 Other pitfalls of posterior wiring techniques are associated with a gradual loss of reduction and pseudoarthrosis.117 In one paper, loss of correction has been reported as high as 34% in 5-year follow-up.53
Klimo et al reported a series of 78 cases with OO suffering from RAAD, 15% of the cases had a history of failed posterior wiring already done by other surgeons and were referred for redo surgery and restabilization.5 Surprisingly, even in the last decade, Visocchi et al showed preference towards advocating posterior wiring technique.61 Parallel to the posterior wiring fixation, C1-C2 stabilization by laminar clamps or claws was introduced in early 1980.118 Out of the 23 reported cases with RAAD, 20 cases due to OO were described by Sumi et al in 1997, in which the patients were treated with different posterior wiring techniques but there have been 3 reported cases implementing the use of laminar clamps.53
Recently, Maciejczak et al119 confirmed that the fusion rate in C1-C2 clawing is comparable to that of various posterior screwing techniques. Despite all odds, these techniques remained the surgery of choice for several decades until transarticular C1-C2 screw fixation was introduced by Magerl and Seeman in 1987.120 It should be noted that transarticular C1-C2 screw procedure is technically demanding and requires precise intraoperative and radiological knowledge of VA anatomy to minimize the risk of iatrogenic injury.
Despite the long duration of the learning curve, the transarticular screw method provides a reasonable and biomechanically superior construct compared to other wiring techniques.10
Good stability achieved by combining transarticular screws and posterior wiring was documented by Grob et al. in 1992.121 High fusion rate was demonstrated using this combination by Dickman and Sonntag in 1998. All the nine cases operated by these surgeons indicated OO with RAAD.114 Later in 2001, Haid et al. successfully used this technique in 75 patients. Nine patients out of these 75 reported cases, had congenital anomalies including OO.122
Transarticular C1-C2 fixations are not only implemented in adults, but have also been described as an appropriate method of treatment in children. In 2005, Gluf et al reported the case of 67 children on whom this technique was performed successfully. Twenty-two of these 67 chidren suffered from RAAD, marking the sequel of OO.123 Most of the patients with symptomatic OO reported by Klimo et al, were treated with transarticular C1-C2 screw fixation, which ultimately gave good results. In this case series, 38 out of 78 reported cases were that of children under the age of 16 years.10,114 The suitability of the application of this technique in children was reconfirmed later by Reilly et al in 2006.124
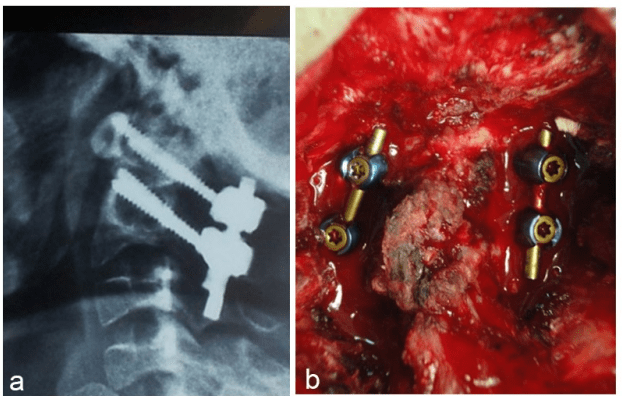
Instead of the posterior wiring technique, additional hooks can be used for the arch of the atlas with a high rate of stability and simplicity. This has been described by Olerud and Olerud in 2001 and later by Wang et al. in 2011.125 It should be noted that, since, there is an observed asymmetry of the facet joints among a large number of patients with OO, this might make transarticular C1-C2 stabilization difficult in this pathology, necessitating an alternative method on the abnormal side.126 About 15 years after the introduction of transarticular C1-C2 technique, posterior C1 lateral mass and C2 pars instrumentation by means of screw-plate was introduced by Goel and Laheri in 1994.127 Thereafter, Harms modified this technique with the application of the polyaxial screw-rod system, which eased and increased stabilization and significantly improved the outcome. In the description of the method by Harms and Melcher,6 (Figure 9) 16 out of 37 patients who underwent this technique had OO, and postoperatively, all showed radiological evidence of fusion. This technique is the mostly used procedure nowadays for reducible OO.10,128,129
Figure 9: Management of OO with Harms technique; (a) lateral radiograph. (b) intraoperative photograph
In 2012, Weng et al reported the case of 19 symptomatic patients with OO who had RAAD. Ten out of 19 reported cases underwent Harms’ procedure, whereas for the remaining 9 patients transarticular C1-C2 supplemented with posterior wiring was used with almost similar radiological and clinical results.64 Brecknell and Malham reported successful C1-C2 internal fixation and fusion for 3 adult patients with OO using polyaxial C1 lateral mass and C2 pedicle screws placed with image guidance.57
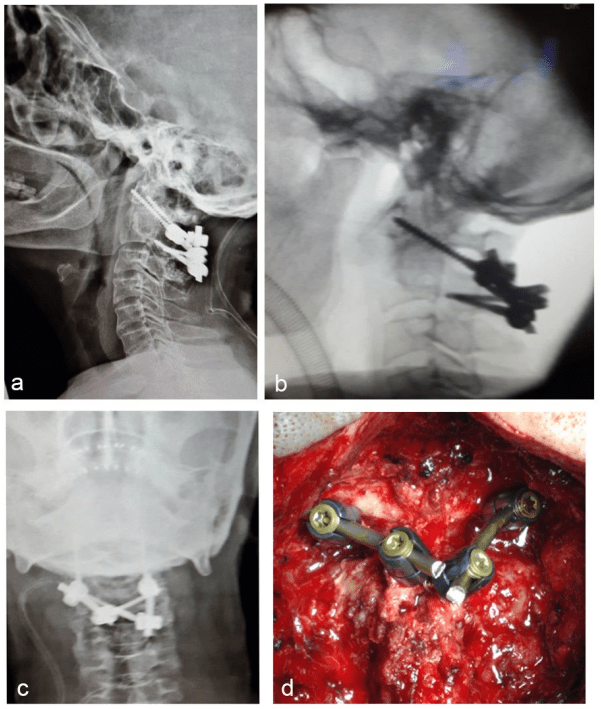
Another alternative technique for C2 pedicle screw, in the presence of overriding vertebral artery, is translaminar screw that can be used on one or both sides to avoid vertebral artery injury (Figure 10).
Figure 10: Translaminar screw technique in OO. (a) AP view, (b) intra-operative fluoroscopy, (c) lateral view, (d) intra-operative photograph.
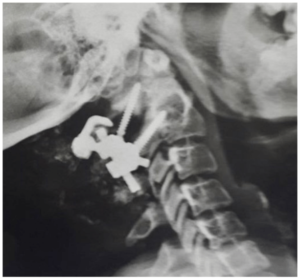
For example, in 2006, Leonard and Wright described the case of 3 patients with symptomatic OO who were successfully treated with this technique. An interesting option instead of lateral mass screw is the use of C1 laminar hook under certain circumstances, where the use of lateral mass screw on one or both sides is not feasible (Figure 11).74
Figure 11: Use of Unilateral atlas hook in a patient with OO
Pediatrics
The management of atlantoaxial instability due to OO in children, albeit the rarity, is a challenging issue due to the smaller size of the spine. Although previous methods of posterior wiring are technically feasible, they might be associated with wire breakage and lower fusion rates, thus necessitating long-term postoperative immobilization in a collar or even in halo vest.
It was expected that the transarticular C1-C2 fixation technique had provided a unique and previously unattainable means of achieving atlantoaxial stabilization in children. The maximum overall rate of complications of transarticular technique had been less than 2% among the pediatric population with a very low rate of vertebral artery injury in the hands of experts. Reilly and Choit published the application of this technique by the implementation of posterior C1-C2 fusion in children with C1-C2 instability. Twelve reported cases were indicative of OO, and 9 of these 12 patients showed symptoms of instability for which transarticular screw fixation was used. It was believed that for all the patients, radiographic bony fusion was achieved such that neither vertebral artery injuries nor other major complications were observed.124 Apart from transarticular C1-C2 fixation, Harms technique facilitated the management of atlantoaxial instability due to OO in this age group. There are several reports demonstrating the usefulness of Harms’ procedure in children.
Similar to transarticular fixation, this procedure does not require the presence of intact posterior elements at C1 or C2. Thus, in this case, the laminectomy of C1 arch is an essential part of surgery, this procedure being the gold standard.
Potential pitfalls associated with the C1 lateral mass screw placement procedure include the injury of C2 nerve root and the vertebral artery where both can be avoided by taking precautions. Autogenous bone chips or allograft mixed with bone marrow aspirate can be used for bony fusion.130 In 2002, Stokes et al introduced the use of C2 lateral mass screw instead of pars screws. In this technique, the entry point is in the inferior midportion of C2 facet joint, similar to the point used in transarticular C1-C2 screw placement. The pilot hole and trajectory are similar to those used for transarticular screws but are directed more caudally and medially to avoid the transverse foramen.
The depth for screw placement ranges between 14-18 mm. After tapping, a 3.5 mm screw of appropriate size is inserted on each side. The depth of the hole or the length of the screw can be estimated with the application of preoperative CT scan. The placement of a rod in the tulips of C1-C2 screws and the nut tightening are the final steps of the procedure.
Although the superiority of all these modern instrumentation techniques over posterior wiring has been confirmed sufficiently in the literature,117 for those with marked instability, posterior wiring technique has a crucial role and can be used in combination with these modern techniques whenever the possibility of failure in those with severe osteoporosis or marked instability exist. Ni et al used C1 laminar hooks and C2 pedicle screws for internal fixation in a series of 13 C1-C2 posterior internal fixation and fusion procedures. OO was the cause of instability in four patients.131
A complete resolution of retro-odontoid soft-tissue mass designated as pseudotumor or panus has been reported with transarticular C1-C2 screw-rod fixation.96,132 Three more case reports have described that retro-odontoid synovial cysts associated with atlantoaxial instability regressed after surgical atlantoaxial fusion without resection of the cyst.98,133 It is hypothesized that posterior fixation of degenerative tissue has the potential to reduce repetitive mechanical stimulation, thereby inhibiting stress on the spinal cord and reducing the inflammation that produces degenerative lesions.
Management of Irreducible Atlantoaxial Instability in OO (IAAD)
An atlantoaxial dislocation is identified as irreducible when the condition is neither corrected in dynamic radiographs nor with cranial traction. In patients with OO, atlantoaxial joint remains stable for a variable time with the support of ligamentous structures. However, daily activities and repetitive flexion movements of the head gradually result in the forward displacement of C1 facet joints on horizontal C2 facet joints. With further anterior slippage of the atlas, it gradually loses the support of the posterior ligamentous complex as well as tectorial membrane. Simultaneously, the remodeling of C1 and C2 facet joints occurs, which facilitate forward translation. The synovial enfolding, scar tissues, and contracture of capsules of C1-C2 facet joints and their remodeling ultimately results in IAAD. Before any surgical attempt for the reduction of the deformity, closed reduction should be tried.
It should be reminded as a rule that, in irreducible conditions, before surgical decision-making, cranial traction should be tried as the first line of treatment. It should be emphasized that, even in long-standing atlantoaxial dislocations, there might be a possibility for reduction by cranial traction or manipulation.
In fact, reducing a deformity by traction confirms that the dislocation is not really “irreducible”. Therefore, preoperative conscious traction is recommended as a useful test that should be tried in all cases with atlantoaxial dislocation presumed to be irreducible in dynamic radiographs. For this purpose, in adults, traction should be started with 2.5 kg, with its gradual increase up to 20 kg in conscious states.
In children, the applied weight varies from 7% of the body weight to a maximum of 4 kg according to Kumar and Nayak.134 Salunke et al have proposed starting with 7% of the body weight and its gradual increment upto 5 kg.126 In adults, conscious traction allows the gradual reduction of C1-C2 subluxation with fewer neurological dysfunctions. If neural impairment occurs, the release of the weight results in a faster resolution of the neurological deficit. Another option for closed reduction is traction under general anesthesia. According to some surgeons, a good number of irreducible conditions can be further reduced with the continuation of traction following general anaesthesia due to the relaxation of the corresponding muscles of the neck. The neurological status of the patient can be estimated with neuromonitoring or a wake-up test. In addition to the traction, direct manual facetal distraction might be an effective way to reduce the dislocation under general anesthesia.11
Furthermore, the response to traction in a conscious patient also has prognostic value. Radiological alignment and clinical improvement achieved after traction suggests that even cervicomedullary myelopathy might be reversible.
History of Surgical Intervention in IAAD
Nonetheless, an OO with subluxation might continue to remain unreduced, despite all the above-mentioned methods. Under such circumstances, surgery poses considerable difficulties due to the highly variable anatomy of the upper cervical spine and the surrounding neurovascular structures, posing challenges to the approach. Surgical intervention in irreducible cases has changed with time. Samuel Mixter was the first to perform surgical intervention for an irreducible OO. Although he could only partially reduce the subluxation, the ultimate outcome was satisfactory.28
Later, the treatment for irreducible OO shifted to decompressive laminectomy of the atlas and enlargement of the foramen magnum. However, because of a continuous ventral cervicomedullary compression, this was less appreciated on account of high morbidity and mortality and was abandoned later. Previous records are indicative of one instance in which the procedure for laminectomy of the C1 arch had been complicated with hematomyelia and death.45 Subsequently, reduction by means of backward pulling of the C1 arch with the aid of strong sublaminar wire or with the use of towel clips was introduced. However, because of untoward dangers such as the possibility of consequential cord injury and C1 laminar fracture, its application became questionable.
Later, most surgeons switched to occipitocervical fusion from C2 to occiput and laminectomy of the C1 arch with limited suboccipital craniectomy for treating AA dislocations, which are refractory to close reduction.
This technique was implemented in the treatment of OO with fixed deformity without any chances of reduction. The rationale behind this mode of surgery was the removal of all compressive tissues at the cervicomedullary junction and enlarged foramen magnum. It should be noted that, despite this strategy, this technique had been associated with failure and infrequently with catastrophic consequences, but it became an accepted surgical mode for several years. Even in recent years, this technique has a few proponents as has been implicated in the cases examined by Ding et al These authors have accomplished occipitocervical stabilization after the enlargement of foramen magnum via atlas arch laminectomy in 12 cases of IAAD secondary to OO, and reported their study in 2012. These studies have indicated good and satisfactory results in the long-term follow-up.107
However, transoral odontoidectomy in separate odontoid was described by Greenberg et al as early as the revolutionized occipitocervical stabilization. However, this combination did not become popular until it was reported by Menezes et al in 1980.135
Thereafter, new corridors for odontoidectomy were described. Parallel to these innovations in odontoidectomy, diversity in surgical techniques for the release of C1-C2 facet joints developed for treating irreducible OO. Through the review of literature, we found that it might be helpful to classify all contemporary techniques used in the management of IAAD due to OO by the implementation of two distinct strategies: (1) resection strategy, which aims at the resection of OO ventrally, and (2) releasing strategy, which focuses on the release of C1-C2 facet joints either anteriorly or posteriorly. However, the decision paradigm should be tailored in an individual basis.
Resection strategy: The removal of OO to get rid of its persistent compressive effect on the cervicomedullary region had always been an option in treating irreducible conditions, particularly in dystopic OO. This can be mediated through the transoral, retropharyngeal, or transnasal corridors. By the application of these techniques, the offending anterior bony mass, redundant ligaments, granulation, and hypertrophic scar tissues should be removed and adequate ventral decompression can be achieved. Transoral odontoidectomy for OO dates back to 1968, when Greenberg et al accomplished this procedure successfully in 2 patients with IAAD due to hypoplastic odontoid.136 In the largest case series reported, 28 out of 134 patients operated by Menezes for OO, had IAAD consequentially causing cervicomedullary compromise in whom a transoral decompressive procedure was accomplished.3 This remained the procedure of choice for many authors such as Salunke et al who have reported their experience in 2005.126 However, to avoid any complication of this corridor, a retropharyngeal route was proposed. Later, Leng et al described the endoscopic endonasal resection of OO with fewer complications in comparison to the former route.18 Magrini et al reported the case of an endoscopic endonasal odontoidectomy in a patient with Down syndrome and OO.17 In 2011, Visocchi et al illustrated their experience with endoscopic assisted microsurgical odontoidectomy in 7 patients among which only one of them had OO.61 In 2011, Gempt et al performed the same procedure in a 52-year-old female with OO.137 Recent studies indicate the contribution of Yen et al who operated on 13 patients suffering from IAAD via transnasal endoscopic odontoidectomy without resection of nasal turbinate. Two out of these 13 reported cases implicated OO. The patients who were reported underwent occipitocervical stabilization thereafter.22
Previous records indicate that the introduction of transoral transpharyngeal resection of OO in the case of IAAD, dates back to the works of Greenberg et al In 1968,136 who recommended occipitocervical stabilization, after performing odontoidectomy. Later, this procedure was performed in a large number of patients with different etiologies. Among the 134 patients on whom the surgery was practiced, irreducible OO causing cervicomedullary compromise occurred in 28 patients who had undergone the transoral transpharyngeal procedure. For several years, this technique was recommended as the best option and the procedure of choice for irreducible conditions affecting atlantoaxial joints, in particular, for dystopic OO. However, because of the drawbacks and morbidities encountered in transoral-transpharyngeal corridor such as local infection, retropharyngeal abscess, meningitis, palatal dehiscence, pharyngeal dehiscence, delayed pharyngeal bleeding, velopalatine incompetence, and persistent hoarseness, the retropharyngeal route for odontoidectomy was recommended. Later, Leng et al introduced the procedure of endoscopic endonasal resection of OO with fewer complications in comparison to the former routes. Recent studies indicate the description of endoscopic endonasal odontoidectomy in a patient with Down syndrome suffering from irreducible IAAD due to OO, as reported by Magrini et al.17 In 2011, Gempt et al performed the same procedure in a 52-year-old female with OO.137 A relatively safer route, endoscopic odontoidectomy via transcervical corridor, was described by Wolinsky et al138 This route is similar to that which has been previously described by Smith and Robinson, with the only difference being that the incision is at a higher cervical region. Through the application of these techniques, the offending anterior bony mass, redundant ligaments, granulation, and hypertrophic scar tissues are removed until adequate ventral decompression has been achieved. In fact, in fixed OO, particularly in dystopic patients, cervicomedullary compromise may not necessarily result only from OO, also, the assimilated atlas or the body of axis might cause further compression. Therefore, performing the resection of the caudal part of the clivus, the arch of the atlas, and the cranial part of axis might become necessary.
Regardless of the corridor used for the resection of OO and other offending tissues, instability of the atlantoaxial joints continue to persist and might result in a worser condition. Therefore, after achieving adequate decompression to avoid serious sequels of further instability, and to allow early mobilization and rehabilitation, a second operation for stabilization becomes mandatory. For this purpose, occipitocervical instrumentation and fusion are recommended by most authors. Nowadays, odontoidectomy for IAAD due to OO has become confined to either dystopic OO, in which is characterized by an apparent non-reducible compression of the foramen magnum, or orthotopic OO, in which the deformity cannot be reduced by interfacet releasing methods.
This paves the path for further research to investigate the significance of odontoidectomy, particularly in the treatment of orthotopic patients using the transoral corridor where, despite the attempts to refine the surgical procedure, risks and complications exist.
Occipitocervical fusion and stabilization have been used in IAAD after performing transoral odontoidectomy for the treatment of OO. This procedure has been used traditionally for RAAD. For example, Behari et al have reported this technique in 109 cases of RAAD.139 The same strategy has been advocated by Jain. Later, Ransford et al reported the invention of contoured rod and used it in combination with bony fixation.140 In recent times, the concept of occipitocervical fixation has revolutionized with the application of advanced sophisticated instruments that are stronger and ensure immediate stability.
Releasing strategy: This strategy directs the application of different challenging procedures either ventrally or posteriorly that aim to clear C1-C2 facet joints from the scar tissues hampering reduction.
Recent studies observe that our understanding of releasing strategies in IAAD has been enhanced by various research contributions in this domain. These releasing methods seem very effective, particularly if we consider that, in longstanding subluxations, failure of reduction is almost certainly due to the damage of the joint capsule, synovial infolding, and interposition or invagination of the granulation tissues into the joint, aggravated by muscle spasm or ligamentous contracture, and even bony fusion.
Despite technical difficulties, the possibility of opening up of the joint, wide removal of the articular cartilage, and manipulation of the facets presents a new dimension to the treatment of irreducible atlantoaxial instability. Besides, the use of intrafacet bone graft facilitates fusion and enhances stability.
The decision to release from the front or the back is dependent on the surgeon’s preference, experience, and comfort level with the particular approach. Nonetheless, after performing any releasing procedure via open reduction, ensuring the stabilization of the corresponding joints is mandatory either in the same session or in a staged manner. It should be noted that direct facet joint opening and its release, is a prerequisite for the completion of all the releasing techniques, either anteriorly or posteriorly.
Anterior releasing methods: The anterior release of the C1-C2 facet joints under skull traction, is an intensive procedure that can be accomplished through transoral, retropharyngeal, endoscopic, endonasal, and transcervical corridors.
The transoral release of the C1-C2 facet joints under skull traction was first performed by Subin et al in 1995, in which the locked joints were released transorally.14 The patient was subjected to skull traction for a few days following the interfacet fusion and the Minerva cast was used to achieve solid fusion.14 Wang et al applied this method and following transoral C1-C2 facet joint release and reduction by traction, performed posterior transarticular C1-C2 screw fixation.16 The transoral route remained the accepted corridor until the retropharyngeal approach for the release of the corresponding joints with 2 sequential posterior internal fixation was introduced for IAAD. This corridor has some proponents such as Hao et al who reported the application of the same technique in 2013.141 Later, Lü et al performed the same technique through the same route but endoscopically in 21 reported cases, of which 7 reported cases were that of OO patients.19 There is further scope for investigating the retropharyngeal or transnasal endoscopic release of C1-C2 facet joints in the future, without encountering the risks associated with the use of the transoral route.
In all the recorded releasing surgeries, stabilization was achieved via the posterior route in the same session or in a staged manner. However, in 2005, Yin et al reported the release of C1-C2 articular joints’ capsule and performed a transoral reduction of atlantoaxial dislocation, but stabilized the joints with a supplementing plate and screw through the same corridor in 5 patients with IAAD, of whom 2 patients reportedly had a dysplastic odontoid. In 2010, Wang et al described transoral one stage anterior C1-C2 joint release followed by the anterior C1-C2 osteosynthesis in a 52-year-old female with OO who was suffering from IAAD. In 2011, Ai et al142 introduced a novel one-stage transoral technique. In their technique, after the resection of the articular capsules, the granulation, and the scar tissues inside the atlantoaxial facet interspace, reduction was achieved with the use of the transoral atlantoaxial reduction plate (TARP). The release of C1-C2 facet joints using the conventional high cervical approach is not impossible but very difficult. Liu et al could accomplish this via high cervical incision and by using conventional self-retaining cervical retractors where the simultaneous retraction of the mandible was maintained with an S-shaped handheld retractor. The corresponding facet release was done with the aid of a fibre-optic endoscope and light source. After the release of the facet joints on both sides, reduction was mediated with traction and manipulation.
Subsequent to alignment, the pathological condition was stabilized via posterior internal fixation. The same technique was employed in 2014 by Ma et al for IAAD.143 However, in 2010, Wu et al could access the corresponding field with the tubular approach.144 The entry incision was high cervical, medical to sternocleidomastoid. A K-wire was aimed at the C1-C2 facet joint, and after enlarging the route with different size dilators, a tubular retractor if fixed in place, via this working channel, the release of the facet joints could be achieved with the aid of an endoscope where reduction became possible with cranial traction. Subsequently, the corresponding joints could be stabilized with the use of percutaneous anterior transarticular screws.
Posterior Releasing Methods
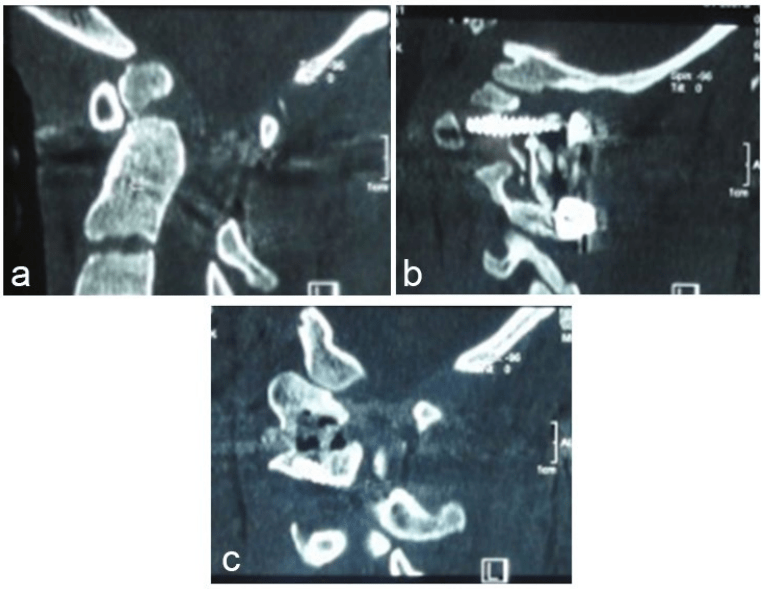
Due to the familiarity of most neurosurgeons with the posterior techniques of reduction, these techniques are more attractive than demanding anterior corridors. One of the most interesting procedures is the technique that has been described by Goel. Accordingly, the atlantoaxial facet joints should be widely exposed on both sides after the sectioning of the C2 ganglion. C1-C2 joints should be opened by direct manual facetal distraction with the aid of a small osteotomy. Later, the reduction by means of Goel plate and screws can be accomplished.127 In this method, after placing a plate of appropriate size on the facet joints of C1 and C2 vertebrae, the plate is fixed by the tightening of the C2 screw first. Later, to pull the C1 back, the corresponding C1 screw is tightened. This is similar to the reduction of spondylolisthesis where sequentional tightening pulls back the second vertebra (Figure 12 and 13).
Figure 12: OO with Irreducible atlantoaxial dislocation,(a) extension view, (b) Flexion view,(c) Pre-Operative reconstructed sagittal C.T. scan., (d) Axial C.T. showing bony formation between C1 and C2

Figure 13: Post Op C.T. scan , (a) Note optimal reduction, (b&c) Note intra-facet arthrodesis after reduction
Taking this method into consideration, the Harms technique with the use of polyaxial screws can be applied. The independent acquisition of C1 lateral mass and C2 pedicle, respectively, indicates that a reduction maneuver can be attempted intraoperatively. For this purpose, after tightening of C2 nuts first, C1 screw can be pulled back by a strong persuader, and with the tightening of the corresponding nut, the atlas can be secured in a reduced position. In 2013, Yin et al reported the reduction of IAAD in a relatively similar maneuver (Figure 12). Their proposed mechanism of reduction is associated with the use of implanted screws and rods between C1 and C2 which act as a lever system, drawing C1 backward and pushing C2 forward after removing circumambient obstruction and scars, and thoroughly releasing the facet joints. In the latter approach, direct manual facetal distraction with the aid of a small osteotome seems as an effective way to release and then reduce the dislocation.
Direct facet joint opening and its release might appear to be a challenging surgical procedure with respect to the direction of the joint in irreducible cases. The presence of a large venous plexus in the lateral gutter makes the procedure difficult and tedious. However, C2 ganglion resection is mandatory in such cases to facilitate exposure of the facet joints. Despite the formidable technical difficulties in surgery, the possibility of opening up of the joint, wide removal of the articular cartilage, and manipulation of the facets presents a new dimension to the treatment of OO.97
Other than this report, a few other reduction techniques in addition to releasing have developed. For example, in the same year, Suh et al introduced a technique where a temporary T-shaped tool was used instead of a rod acting as a lever to pull back the C1 lateral mass screw. In 2011, Liu et al described an advanced technique named posterior cable-dragged reduction/cantilever beam internal fixation surgery, where the atlas arm is pulled back with the aid of a cable tightened on the U-shaped rod that is already attached to bilateral C2-C3 lateral mass screws.25
Another interesting method is the application of the posterior rotating rod strategy introduced by Chang-Wei et al In this method, C1 lateral mass screws and C2 and C3 pedicle screws are inserted at appropriate points; subsequently, a curved rod with its convex side placed posteriorly is introduced in the tulips of the screws, and the nuts are tightened, but not fully. Then, two rod rotators are used such that both rods are rotated simultaneously until the rod is placed in a lordotic position.97
Prognosis
Poor observations are associated with chronic patients who are in a poor preoperative grade. Concomitant gliosis of the nuclei at the cervicomedullary junction, and demyelination of long tracts at this region due to repeated trauma and hypoxia due to venous stasis, have been implicated as the probable causes of poor outcome after surgery. However, a small percentage of patients with marked disability with either IAAD or RAAD might benefit from proper surgery and show good clinical results. This is particularly observed among patients who have developed quadriparesis in a short period of time. A case reported in 1974 by Rezaian is a good example of a related study.145 Shortened duration of myelopathy indicates better results with surgery rather than more chronic cases of myelopathy. Under such clinical emergencies, it is imperative to increase the spinal canal diameter at the CVJ.
ACKNOWLEDGMENTS
The authors appreciate Drs. Asgari and Alireza Mojtahed for their assistance in the preparation of this review article.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.


















