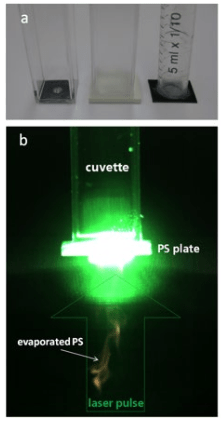
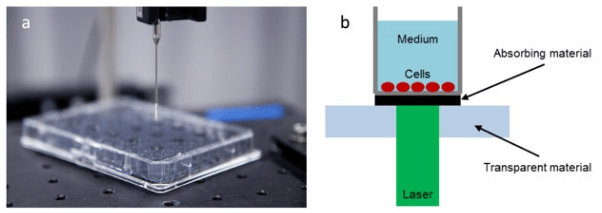
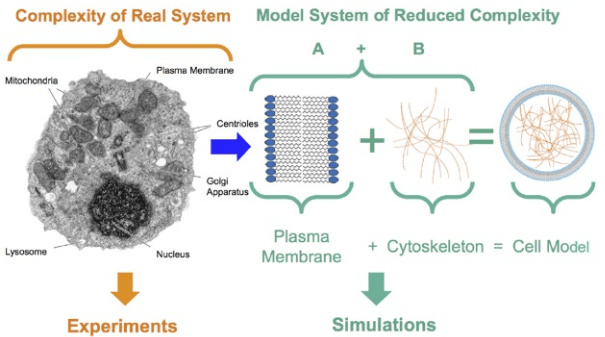
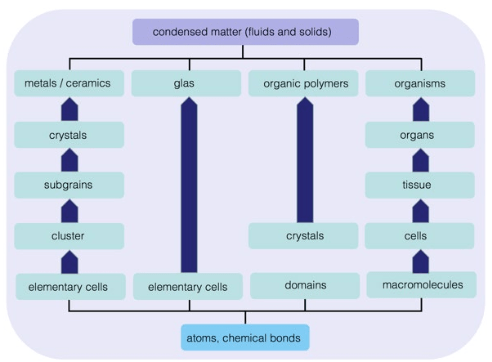
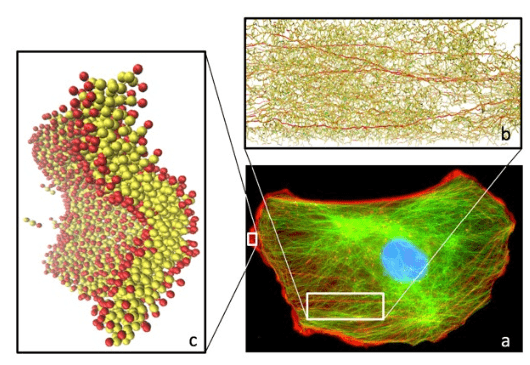
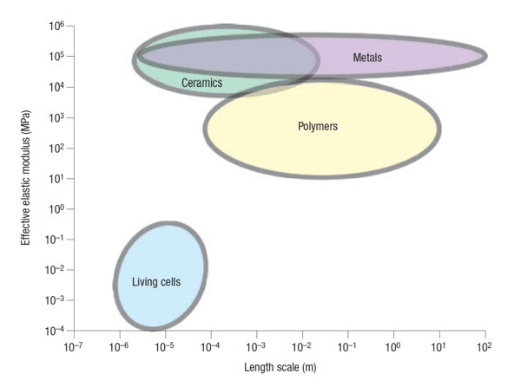
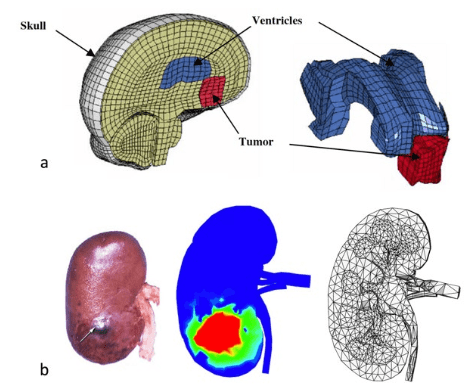
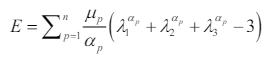1. Mendelsohn J, Howley PM, Israel MA, Gray JW, Thompson CB. The Molecular Basis of Cancer. Philadelphia, PA, USA: Elsevier Science; 2014.
2. Blagosklonny MV, Robey R, Bates S, Fojo T. Pretreatment with DNA-damaging agents permits selective killing of checkpoint-deficient cells by microtubule-active drugs. J Clin Invest. 2000; 105(4): 533-539. doi: 10.1172/JCI8625
3. Pearson OH, Manni A, Arafah BM. Antiestrogen treatment of breast cancer: An overview. Cancer Res. 1982; 42(8 Suppl): 3424s-3429s. Web site. http://cancerres.aacrjournals.org/content/42/8_Supplement/3424s.short. Accessed July 24, 2016
4. Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007; 3(4): 413-438. doi: 10.1016/j.actbio.2007.04.
002
5. Guck J, Schinkinger S, Lincoln B, et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005; 88(5): 3689-3698. doi: 10.1529/biophysj.104.045476
6. Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992; 256(5064): 1677-1680. doi: 10.1126/science.256.5064.1677
7. Gardel ML, Valentine MT, Crocker JC, Bausch AR, Weitz DA. Microrheology of entangled F-actin solutions. Phys Rev Lett. 2003; 91(15): 158302-158302. doi: 10.1103/PhysRevLett.91.158302
8. Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Devel Biol. 2009; 26: 315-333. doi: 10.1146/annurev.cellbio.011209.122036
9. Dunn BB. Cancer: Solving an age-old problem. Nature. 2012; 483(7387): S2-S6. doi: 10.1038/483S2a
10. Lackey L, Peterson C, Barr RG. Contrast-enhanced ultrasound-guided radiofrequency ablation of renal tumors. Ultrasound Q. 2012; 28(4): 269-274. doi: 10.1097/RUQ.0b013e318274de66
11. Goyal J, Verma P, Sidana A, Georgiades CS, Rodriguez R. Single center comparative oncologic outcomes of surgical and percutaneous cryoablation for treatment of renal tumors. J Endourol. 2012; 26(11): 1413-1419. doi: 10.1089/end.2012.0244
12. Vogl TJ, Straub R, Eichler K, Söllner O, Mack MG. Colorectal carcinoma metastases in liver: Laser-induced interstitial thermotherapy-local tumor control rate and survival data. Radiology. 2004; 230(2): 450-458. doi: 10.1148/radiol.2302020646
13. Suhr F, Delhasse Y, Bungartz G, Schmidt A, Pfannkuche K, Bloch W. Cell biological effects of mechanical stimulations generated by focused extracorporeal shock wave applications on cultured human bone marrow stromal cells. Stem Cell Res. 2013; 11(2): 951-964. doi: 10.1016/j.scr.2013.05.010
14. Carome EF, Clark NA, Moeller CE. Generation of acoustic signals in liquids by ruby laser-induced thermal stress transients. Appl Phys Lett. 1964; 4(6): 95-97. doi: 10.1063/1.1753985
15. Krehl POK. History of Shock Waves, Explosions and Impact: A Chronological and Biographical Reference. Berlin, Germany: Springer; 2009.
16. Steinhauser MO, Hiermaier S. A review of computational methods in materials science: Examples from shock-wave and polymer physics. Int Mol Sci. 2009; 10(12): 5135-5216. doi: 10.3390/ijms10125135
17. Rodriguez V, Saurel R, Jourdan G, Houas L. Solid-particle jet formation under shock-wave acceleration. Phys Rev E Stat Nonlin Soft Matter Phys. 2013; 88(6): 063011. doi: 10.1103/PhysRevE.88.063011
18. Zheng J, Chen QF, Gu YJ, Chen ZY. Hugoniot measurements of double-shocked precompressed dense xenon plasmas. Phys Rev E Stat Nonlin Soft Matter Phys. 2012; 86(6): 066406. doi: 10.1103/PhysRevE.86.066406
19. Falk K, Regan SP, Vorberger J, et al. Comparison between x-ray scattering and velocity-interferometry measurements from shocked liquid deuterium. Phys Rev E Stat Nonlin Soft Matter Phys. 2013; 87(4): 043112. doi: 10.1103/PhysRevE.87.043112
20. Brujan EA, Ikeda T, Matsumoto Y. Jet formation and shock wave emission during collapse of ultrasound-induced cavitation bubbles and their role in the therapeutic applications of high-intensity focused ultrasound. Phys Med Biol. 2005; 50(20): 4797-4809. doi: 10.1088/0031-9155/50/20/004
21. Iakovlev S, Iakovlev S, Buchner C, et al. Resonance-like phenomena in a submerged cylindrical shell subjected to two consecutive shock waves: The effect of the inner fluid. J Fluids Struct. 2014; 50: 153-170. doi: 10.1016/j.jfluidstructs.2014.05.013
22. Bringa EM, Caro A, Wang YM, et al. Ultrahigh strength in nanocrystalline materials under shock loading. Science. 2005; 309(5742): 1838-1841. doi: 10.1126/science.1116723
23. Kadau K, Germann TC, Lomdahl PS, et al. Shock waves in polycrystalline iron. Phys Rev Lett. 2007; 98(13): 135701. doi: 10.1103/PhysRevLett.98.135701
24. Knudson MD, Desjarlais MP, Dolan DH. Shock-wave exploration of the high-pressure phases of carbon. Science. 2008; 322(5909): 1822-1825. doi: 10.1126/science.1165278
25. Gurnett DA, Kurth WS. Electron plasma oscillations upstream of the solar wind termination shock. Science. 2005; 309(5743): 2025-2027. doi: 10.1126/science.1117425
26. Gurnett DA, Kurth WS. Intense plasma waves at and near the solar wind termination shock. Nature. 2008; 454(7200): 78-80. doi: 10.1038/nature07023
27. Dutton Z, Budde M, Slowe C, Hau LV. Observation of quantum shock waves created with ultra-compressed slow light pulses in a Bose-Einstein condensate. Science. 2001; 293(5530): 663-668. doi: 10.1126/science.1062527
28. Damski B. Shock waves in a one-dimensional Bose gas: From a Bose-Einstein condensate to a Tonks gas. Phys Rev A. 2006; 73(4): 043601. doi: 10.1103/physreva.73.043601
29. Chang JJ, Engels P, Hoefer MA. Formation of dispersive shock waves by merging and splitting Bose-Einstein condensates. Phys Rev Lett. 2008; 101(17): 170404. doi: 10.1103/PhysRevLett.101.170404
30. Millot M, Dubrovinskaia N, Černok A, et al. Planetary science. Shock compression of stishovite and melting of silica at planetary interior conditions. Science. 2015; 347(6220): 418-420. doi: 10.1126/science.1261507
31. McKee CF, Draine BT. Interstellar shock waves. Science. 1991; 252(5004): 397-403. doi: 10.1126/science.252.5004.397
32. McClure S, Dorfmüller C. Extracorporeal shock wave therapy: Theory and equipment. Clin Tech Equine Pract. 2002; 2(4): 348-357. doi: 10.1053/j.ctep.2004.04.008
33. Lingeman JE, McAteer JA, Gnessin E, Evan AP. Shock wave lithotripsy: Advances in technology and technique. Nat Rev Urol. 2009; 6(12): 660-670. doi: 10.1038/nrurol.2009.216
34. Cherenkov PA. Visible emission of clean liquids by action of gamma radiation. R Dokl Akad Nauk SSSR. 1934; 2: 451-454. doi: 10.1234/12345678
35. Rassweiler JJ, Knoll T, Kohrmann KU, et al. Shock wave technology and application: An update. Eur Urol. 2011; 59(5): 784-796. doi: 10.1016/j.eururo.2011.02.033
36. Coleman AJ, Saunders JE. A review of the physical properties and biological effects of the high amplitude acoustic fields used in extracorporeal lithotripsy. Ultrasonics. 1993; 31(2): 75-89. doi: 10.1016/0041-624X(93)90037-Z
37. McAteer JA, Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol. 2008; 28(2): 200-213. doi: 10.1016/j.semnephrol.2008.01.003
38. Brümmer F, Bräuner T, Hülser DF. Biological effects of shock waves. World J Urol. 1990; 8(4): 224-232. doi: 10.1007/BF01580025
39. Delius M. Medical applications and bioeffects of extracorporeal shock waves. Shock Waves. 1994; 4(2): 55-72. doi: 10.1007/BF01418569
40. Oosterhof GO, Smits GA, De Ruyter AE, Schalken JA, Debruyne FM. Effects of high-energy shock waves combined with biological response modifiers in different human kidney cancer xenografts. Ultrasound Med Biol. 1991; 17(4): 391-399. doi: 10.1016/0301-5629(91)90139-N
41. Gamarra F, Spelsberg F, Dellian M, Goetz AE. Complete local tumor remission after therapy with extra-corporeally applied high-energy shock waves (HESW). Int J Cancer. 1993; 55(1): 153-156. doi: 10.1002/ijc.2910550127
42. Lauer U, Bürgelt E, Squire Z, et al. Shock wave permeabilization as a new gene transfer method. Gene Ther. 1997; 4(7): 710-715. doi: 10.1038/sj.gt.3300462
43. Bao S, Thrall BD, Gies RA, Miller DL. In vivo transfection of melanoma cells by lithotripter shock waves. Cancer Res. 1998; 58(2): 219-221. Web site. http://cancerres.aacrjournals.org/content/58/2/219.short. Accessed July 24, 2016
44. Delius M, Adams G. Shock wave permeabilization with ribosome inactivating proteins: A new approach to tumor therapy. Cancer Res. 1999; 59(20): 5227-5232. Web site. http://cancerres.aacrjournals.org/content/59/20/5227.long. Accessed July 24, 2016
45. Tschoep K, Hartmann G, Jox R, et al. Shock waves: A novel method for cytoplasmic delivery of antisense oligonucleotides. J Mol Med. 2001; 79(5-6): 306-313. doi: 10.1007/s001090100204
46. Kodama T, Doukas AG, Hamblin MR. Delivery of ribosome-inactivating protein toxin into cancer cells with shock waves. Cancer Lett. 2003; 189(1): 69-75. doi: 10.1016/S0304-3835(02)00503-7
47. Michel MS, Erben P, Trojan L, et al. Acoustic energy: a new transfection method for cancer of the prostate, cancer of the bladder and benign kidney cells. Anticancer Res. 2004; 24(4): 2303-2308. Web site. http://ar.iiarjournals.org/content/24/4/2303.long. Accessed July 24, 2016
48. Ohl C-D, Wolfrum B. Detachment and sonoporation of adherent HeLa-cells by shock wave-induced cavitation. Biochim Biophys Acta. 2003; 1624(1-3): 131-138. doi: 10.1016/j.bbagen.2003.10.005
49. Ohl C-D, Arora M, Ikink R, et al. Sonoporation from jetting cavitation bubbles. Biophys J. 2006; 91(11): 4285-4295. doi: 10.1529/biophysj.105.075366
50. Moosavi-Nejad SF, Hosseini SMR, Satoh M, Takayama K. Shock wave induced cytoskeletal and morphological deformations in a human renal carcinoma cell line. Cancer Sci. 2006; 97(4): 296-304. doi: 10.1111/j.1349-7006.2006.00172.x
51. Bushnell JC, McCloskey DJ. Thermoelastic stress production in solids. J Appl Phys. 1968; 39(12): 5541-5546. doi: 10.1063/1.1656010
52. Yang LC. Stress waves generated in thin metallic films by a Q-switched ruby laser. 1974; 45(6): 2601-2608. doi: 10.1063/1.1663636
53. Fabbro R, Fournier J, Ballard P, Devaux D, Virmont J. Physical study of laser-produced plasma in confined geometry. J Appl Phys. 1990; 68(2): 775-784. doi: 10.1063/1.346783
54. Venugopalan V, Deutsch TF. Stress generated in polyimide by excimer-laser irradiation. J Appl Phys. 1993; 74(6): 4181-4189. doi: 10.1063/1.354422
55. Doukas AG, Flotte TJ. Physical characteristics and biological effects of laser-induced stress waves. Ultrasound Med Biol. 1996; 22(2): 151-164. doi: 10.1016/0301-5629(95)02026-8
56. Yao C-P, Zhang Z-X, Rahmanzadeh R, Huettmann G. Laser-based gene transfection and gene therapy. IEEE Trans Nanobiosci. 2008; 7(2): 111-119. doi: 10.1109/TNB.2008.2000742
57. Doukas AG, Kollias N. Transdermal drug delivery with a pressure wave. Adv Drug Deliver Rev. 2004; 56(5): 559-579. doi: 10.1016/j.addr.2003.10.031
58. Brümmer F, Brenner J, Bräuner T, Hülser DF. Effect of shock waves on suspended and immobilized L1210 cells. Ultrasound Med Biol. 1989; 15(3): 229-239. doi: 10.1016/0301-5629(89)90067-7
59. de Reijke TM, Schamhart DH, Kurth KH, Löwik CW, Donkers LH, Sterenborg HJ. Tumor growth delay by laser-generated shock waves. Lasers Surg Med. 1994; 14(3): 205-212. doi: 10.1002/lsm.1900140302
60. Doukas AG, McAuliffe DJ, Lee S, Venugopalan V, Flotte TJ. Physical factors involved in stress-wave-induced cell injury: The effect of stress gradient. Ultrasound Med Biol. 1995; 21(7): 961-967. doi: 10.1016/0301-5629(95)00027-O
61. Lee S, Anderson T, Zhang H, Flotte TJ, Doukas AG. Alteration of cell membrane by stress waves in vitro. Ultrasound Med Biol. 1996; 22(9): 1285-1293. doi: 10.1016/S0301-5629(96)00149-4
62. Douki T, Lee S, Dorey K, Flotte TJ, Deutsch TF, Doukas AG. Stress-wave-induced injury to retinal pigment epithelium cells in vitro. Lasers Surg Med. 1996; 19(3): 249-259. doi: 10.1002/(SICI)1096-9101(1996)19:3<249::AID-LSM1>3.0.CO;2-S
63. Huber PE, Jenne J, Debus JJ, Wannenmacher MF, Pfisterer P. A comparison of shock wave and sinusoidal-focused ultrasound-induced localized transfection of HeLa cells. Ultrasound Med Biol. 1999; 25(9): 1451-1457. doi: 10.1016/S0301-5629(99)00099-X
64. Kohri K, Uemura T, Iguchi M, Kurita T. Effect of high energy shock waves on tumor cells. Urol Res. 1990; 18(2): 101-105. doi: 10.1007/BF00302468
65. Mulholland SE, Lee S, McAuliffe DJ, Doukas AG. Cell loading with laser-generated stress waves: The role of the stress gradient. Pharm Res. 1999; 16(4): 514-518. doi: 10.1023/A:1018814911497
66. Lee S, Doukas AG. Laser-generated stress waves and their effects on the cell membrane. IEEE J Sel Top Quant. 1999; 5(4): 997-1003. doi: 10.1109/2944.796322
67. Kato M, Ioritani N, Suzuki T, et al. Mechanism of anti-tumor effect of combination of bleomycin and shock waves. Jpn J Cancer Res. 2000; 91(10): 1065-1072. doi: 10.1111/j.1349-7006.2000.tb00886.x
68. Sondén A, Svensson B, Roman N, Brismar B, Palmblad J, Kjellstöm BT. Mechanisms of shock wave induced endothelial cell injury. Lasers Surg Med. 2002; 31(4): 233-241. doi: 10.1002/lsm.10093
69. Kodama T, Kodama T, Hamblin MR, Hamblin MR, Doukas AG. Cytoplasmic molecular delivery with shock waves: Importance of impulse. Biophys J. 2000; 79(4): 1821-1832. doi: 10.1016/S0006-3495(00)76432-0
70. Juhasz T, Hu XH, Turi L, Bor Z. Dynamics of shock waves and cavitation bubbles generated by picosecond laser pulses in corneal tissue and water. Lasers Surg Med. 1993; 15(1): 91-98. doi: 10.1002/lsm.1900150112
71. Strand OT, Goosman DR, Martinez C, Whitworth TL, Kuhlow WW. Compact system for high-speed velocimetry using heterodyne techniques. Rev Sci Instrum. 2006; 77(8): 083108. doi: 10.1063/1.2336749
72. Schmidt M, Kahlert U, Wessolleck J, et al. Characterization of a setup to test the impact of high-amplitude pressure waves on living cells. Nature Sci Rep. 2014; 4: 3849. doi: 10.1038/srep03849
73. Steinhauser MO, Steinhauser MO, Schmidt M. Destruction of cancer cells by laser-induced shock waves: Recent developments in experimental treatments and multiscale computer simulations. Soft Matter. 2014; 10(27): 4778-4788. doi: 10.1039/c4sm00407h
74. Fung YC. Biomechanics: Motion, Flow, Stress, and Growth. New York, USA: Springer; 1990.
75. Verdier C. Rheological Properties of Living Materials. From Cells to Tissues. J Theoretical Medicine. 2003; 5(2): 67-91. doi: 10.1080/10273360410001678083
76. de Gennes PG. Scaling Concepts in Polymer Physics. Ithaca, London, UK; Cornell University Press; 1979.
77. Steinhauser MO. Computational Multiscale Modeling of Fluids and Solids – Theory and Applications. 2nd ed. New York, USA: Springer; 2016.
78. Steinhauser MO. A molecular dynamics study on universal properties of polymer chains in different solvent qualities. Part I. A review of linear chain properties. J Chem Phys. 2005; 122(9): 094901. doi: 10.1063/1.1846651
79. Bienfait Y. Modelling and molecular dynamics simulations of flexible polymer networks. In: Steinhauser MO, ed. December 2013.
80. Schindler T, Kröner D, Steinhauser MO. On the dynamics of molecular self-assembly and the structural analysis of bilayer membranes using coarse-grained molecular dynamics simulations. Biochim Biophys Acta. 2016; 1858(9): 1955-1963. doi: 10.1016/j.bbamem.2016.05.014
81. Doi M, Edwards SF. The Theory of Polymer Dynamics. New york, USA: Clarendon Press, Oxford; 1986.
82. Bird RB, Hassager O, Armstrong RC, Curtis CF. Dynamics of Polymeric Liquids. New York, USA: Wiley; 1977.
83. Larson RG. The Structure and Rheology of Complex Fluids. New York, USA: Oxford University Press; 1999.
84. Winter HH. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J Rheol. 1986; 30(2): 367. doi: 10.1122/1.549853
85. Flory PJ. Statistical Mechanics of Chain Molecules. New Jersey, USA: John Wiley & Sons, Inc; 1969. doi: 10.1002/bip.1969.360080514
86. Einstein A. Eine neue Bestimmung der Moleküldimensionen. Ann Phys. 1906; 324(2): 289-306. doi: 10.1002/andp.19063240204
87. Oldroyd JG. The elastic and viscous properties of emulsions and suspensions. Science. 1953; 218(1132): 122-132. doi: 10.1098/rspa.1953.0092
88. Hashin Z, Rosen BW. The elastic moduli of fiber-reinforced materials. J Appl Mech. 1964; 31(2): 223-232. doi: 10.1115/1.3629590
89. Hill CR. Theory of mechanical properties of fibre-strengthened materials: I. Elastic behaviour. J Mech Phys Solids. 1964; 12(4): 199-212. doi: 10.1016/0022-5096(64)90019-5
90. Fung YC. Microrheology and constitutive equation of soft tissue. Biorheology. 1988; 25(1-2): 261-270. Web site. http://europepmc.org/abstract/med/3196823. Accessed July 24, 2016
91. Choy TC. Effective Medium Theory: Principles and Applications. Oxford, UK: Oxford University Press; 1999. doi: 10.1093/acprof:oso/9780198705093.001.0001
92. Ingber DE, Heidemann SR, Lamoureux P, Buxbaum RE. Opposing views on tensegrity as a structural framework for understanding cell mechanics. J Appl Physiol. 2000; 89(4): 1663-1678. doi: 10.1016/0012-1606(71)90073-X
93. Stamenovic D, Ingber DE. Models of cytoskeletal mechanics of adherent cells. Biomech Model Mechan. 2002; 1(1): 95-108. doi: 10.1007/s10237-002-0009-9
94. Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003; 116(Pt 7): 1157-1173. doi: 10.1242/jcs.00359
95. Wang N, Naruse K, Stamenovic D, et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci. 2001; 98(14): 7765-7770. doi: 10.1073/pnas.141199598
96. Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys. 2014; 77(4): 046603. doi: 10.1088/0034-4885/77/4/046603
97. Pritchard RH, Huang YYS, Terentjev EM. Mechanics of biological networks: From the cell cytoskeleton to connective tissue. Soft Matter. 2014; 10(12): 1864-1884. doi: 10.1039/c3sm52769g
98. Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in neoplastic disorganization of tissue architecture. PNAS. 1981; 78(6): 3901-3905. Web site. http://www.pnas.org/content/78/6/3901.short. Accessed July 24, 2016
99. Chen D, Wen Q, Janmey PA. Rheology of soft materials. Condensed Matter Physics. 2010; 1: 301-322. doi: 10.1146/annurev-conmatphys-070909-104120
100. Müller KW, Bruinsma RF, Lieleg O, Bausch AR, Wall WA, Levine AJ. Rheology of semiflexible bundle networks with transient linkers. Phys Rev Lett. 2014; 112(23): 238102-238102. doi: 10.1103/PhysRevLett.112.238102
101. Brunner C, Niendorf A, Käs JA. Passive and active single-cell biomechanics: A new perspective in cancer diagnosis. Soft Matter. 2009; 5(11): 2171-2178. doi: 10.1039/b807545j
102. Sayed El T, Mota A, Fraternali F, Ortiz M. A variational constitutive model for soft biological tissues. J Biomech. 2008; 41(7): 1458-1466. doi: 10.1016/j.jbiomech.2008.02.023
103. Qin Z, Dimas L, Adler D, Bratzel G, Buehler MJ. Biological materials by design. J Phys Condens Matter. 2014; 26(7): 073101. doi: 10.1088/0953-8984/26/7/073101
104. Meyers MA, Chen P-Y, Lopez MI, Seki Y, Lin AYM. Biological materials: A materials science approach. J Mech Behav Biomed. 2011; 4(5): 626-657. doi: 10.1016/j.jmbbm.2010.08.005
105. Ogden RW. Large Deformation Isotropic Elasticity – On the Correlation of Theory and Experiment for Incompressible Rubberlike Solids. Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences. 1972; 326(1567): 565-584. doi: 10.1098/rspa.1972.0026
106. Mooney M. A theory of large elastic deformation. J Appl Phys. 1940; 11(9): 582-592. doi: 10.1063/1.1712836
107. Rivlin RS. Large Elastic Deformations of Isotropic Materials. IV. Further Developments of the General Theory. Philosophical Transactions of the Royal Society of London Series A Mathematical and Physical Sciences. 1948; 241(8): 379-397. doi: 10.1098/rsta.1948.0024
108. Miller K, Chinzei K. Mechanical properties of brain tissue in tension. J Biomech. 2002; 35(4): 483-490. doi: 10.1016/S0021-9290(01)00234-2
109. Xu M, Nowinski WL. Talairach-Tournoux brain atlas registration using a metalforming principle-based finite element method. Med Image Anal. 2001; 5(4): 271-279. doi: 10.1016/S1361-8415(01)00045-7
110. Warfield SK, Haker SJ, Talos I-F, et al. Capturing intraoperative deformations: research experience at Brigham and Women’s Hospital. Med Image Anal. 2005; 9(2): 145-162. doi: 10.1016/j.media.2004.11.005
111. Miller K, Chinzei K. Constitutive modelling of brain tissue: Experiment and theory. J Biomech. 1997; 30(11-12): 1115-1121. doi: 10.1016/S0021-9290(97)00092-4
112. Miller K. Method of testing very soft biological tissues in compression. J Biomech. 2005; 38(1): 153-158. doi: 10.1016/j.jbiomech.2004.03.004
113. Wittek A, Kikinis R, Warfield SK, Miller K. Brain shift computation using a fully nonlinear biomechanical model. Med Image Comput Comput Assist Interv. 2005; 8(Pt 2): 583-590. doi: 10.1007/11566489_72
114. Wittek A, Hawkins T, Miller K. On the unimportance of constitutive models in computing brain deformation for image-guided surgery. Biomech Model Mechan. 2008; 8(1): 77-84. doi: 10.1007/s10237-008-0118-1
115. Dumpuri P, Thompson RC, Dawant BM, Cao A, Miga MI. An atlas-based method to compensate for brain shift: Preliminary results. Med Image Anal. 2007; 11(2): 128-145. doi: 10.1016/j.media.2006.11.002
116. Bathe KJ. Finite Element Procedures in Engineering Analysis. Cambridge, UK: Prentice Hall; 1982.
117. Cash DM, Miga MI, Sinha TK, Galloway RL, Chapman WC. Compensating for intraoperative soft-tissue deformations using incomplete surface data and finite elements. IEEE Trans Med Imaging. 2005; 24(11): 1479-1491. doi: 10.1109/TMI.2005.855434
118. Kim JS, Sun SX. Continuum modeling of forces in growing viscoelastic cytoskeletal networks. J Theor Biol. 2009; 256(4): 596-606. doi: 10.1016/j.jtbi.2008.10.023
119. Kim S-H, Suh HS, Cho MH, Lee SY, Kim T-S. Finite element simulation of ultrasound propagation in bone for quantitative ultrasound toward the diagnosis of osteoporosis. Conf Proc IEEE Eng Med Biol Soc. 2009; 2009: 436-439. doi: 10.1109/IEMBS.2009.5334707
120. Hostettler A, George D, Rémond Y, Nicolau SA, Soler L, Marescaux J. Bulk modulus and volume variation measurement of the liver and the kidneys in vivo using abdominal kinetics during free breathing. Comput Meth Prog Bio. 2010; 100(2): 149-157. doi: 10.1016/j.cmpb.2010.03.003
121. Cleveland RO, Lifshitz DA, Connors BA, Evan AP, Willis LR, Crum LA. In vivo pressure measurements of lithotripsy shock waves in pigs. Ultrasound Med Biol. 1998; 24(2): 293-306. doi: 10.1016/S0301-5629(97)00270-6
122. Schwartz J-M, Denninger M, Rancourt D, Moisan C, Laurendeau D. Modelling liver tissue properties using a non-linear visco-elastic model for surgery simulation. Med Image Anal. 2005; 9(2): 103-112. doi: 10.1016/j.media.2004.11.002
123. Nava A, Mazza E, Kleinermann F, Avis NJ, McClure J. Determination of the mechanical properties of soft human tissues through aspiration experiments. Medical Image Computing and Computer-Assisted Intervention. 2003; 2003: 222-229. doi: 10.1007/978-3-540-39899-8_28
124. Ottensmeyer MP. Minimally invasive instrument for in vivo measurement of solid organ mechanical impedance. Web site. https://dspace.mit.edu/handle/1721.1/8858. Accessed July 24, 2016
125. Zhu Y, Dong Z, Wejinya UC, Jin S, Ye K. Determination of mechanical properties of soft tissue scaffolds by atomic force microscopy nanoindentation. J Biomech. 2011; 44(13): 2356-2361. doi: 10.1016/j.jbiomech.2011.07.010