INTRODUCTION
The general goal of ocular health screenings is the early detection of what could lead to vision threatening diseases (VTD) such as; age-related macular degeneration (AMD), cataracts, diabetic retinopathy (DR) and glaucoma. An individual that is affected with diabetes (Type 1 or 2) might also be susceptible in developing glaucoma and cataracts. Type 1 diabetes mellitus (T1DM) is an autoimmune disorder, mostly affecting children but can also be diagnosed later in life.1 T1DM results in the destruction of pancreatic β-cells that produce insulin. It has been reported that over 5% of the children worldwide,2 which is about 95 million children, are affected by T1DM. This number is expected to grow and the children will remain on daily self-administration of fast and slow acting insulin until a cure is found. T1DM not only affects the glucose levels in the body but also affects various organs such as kidneys as well as extremities such as nerve end damage to feet. Some of the effects of diabetes T1DM can include: weight loss, infections, slow healing of cuts and wounds, headaches, confusion, fatigue, blurry vision, blindness and coma.3 These interconnected issues have a toll on the person’s life and their loved ones. Left poorly controlled, the life expectancy can be shortened by as much as 12-years, hence, the importance of re-enforcement in diabetes care education and a well maintained daily program. The importance of yearly ocular screenings with careful daily self-monitoring of glucose and HbA1c levels play an important role in safe glucose levels.4 A normal range of fasting blood glucose level is in between 90 and 110 mg/dL prior to having a meal is a target that people without diabetes can maintain.5 The ideal range of HbA1c is <7%.6 When HbA1c reaches levels of >10 % over time, it can lead todamages of the blood vessels in the retina that can lead to proliferative diabetic retinopathy. Conversely, when the blood sugar is too low, it could lead to seizures and could also be life-threatening. A blood glucose of <20 mg/dL can result in a diabetic coma that can quickly lead to the demise of the person.
METHODS
For the past 10-years, the Institute of Ophthalmology and Visual Science, Rutgers New Jersey Medical School, New Jersey, USA, along with other groups holds screenings at the Children with Diabetes (www.childrenwithdiabetes.com) during the week of July 4th. The purpose of the conference is to share knowledge and new developments and technologies with individuals affected with T1DM and their families (parents, siblings, grandparents as well as friends of T1DM participants) about all daily life aspects associated with diabetes and provide them with the latest technologies on how to best control their diabetes. On average, 2,200 individuals attend the conference every year. Of the many events provided during this week-long event is a comprehensive eye wellness that consists of an ocular screening using the latest technology available in remote ophthalmic telemedicine. Sixteen (16) volunteer screeners made up of second year medical students, allied health care specialist, physician, optometrist and ophthalmologist participated (Figure 1) that made up a team. Participants had to pre-register for an appointment to avail them of this screening. Under 18-years-old children had to attend the screening with a parent or legal guardian while 18 year-old and over could be screened without the presence of a parent. Over a four (4) day period, 216 individuals (140 females and 76 males) were screened upon obtaining a consent form ranging from 2 to 74-years-old with an average age of 21-years-old.
Figure 1. CWD 2017 team: Subhashini Chandrasekaran BS, Bernard Szirth PhD, Melissa Notis BS, Daniel Camras BS, Hadeel Sadek BS, Kelly Soules MSc, Somnath Rao BS, Stacey Pan BS, Kim Duong OD, Jim Stroud CRNA, Kimball Dunlap RN, Rob Freund RN, Chris Deleney RN, Hunter Cope SRN, Belinda Peach, Brandy Schmidt, and Laura Billetdeaux.
Not pictured: Peter Khouri, Christopher Khouri, Albert Khouri MD, Jeff Hitchcock
Photo Credit: Chris Kakol, Corporate Image Photography

Figure 2 demonstrates the room flows of the screening process where participants underwent the screening process that averaged 55-minutes including interpretation of dataset. No personal identifiers such as first and last name of participants were used to insure maximum privacy.
Figure 2 (Left and Right). Flow Chart Depicting Patient Flow During Screening. Room on the Left: Patient Baseline Information; Pupil Size, Visual Acuity, Blood Pressure, Pulse, and Patient Intake Form Consisting of Height, Weight, Bmi, Diabetes Management and History, Family and Past Medical and Ocular History. Room on The Right: Retinal Screening Room. 1) Auto-Refractor (Canon, Rkf2 Tokyo, Japan), 2) Tonometer (Canon, Tx 20 Tokyo, Japan), 3) Oct-A (Optovue, Ivue, Fremont, California), and 4) Retinal Camera (Canon, Cr2 Plus-Af With Eos-60d Tokyo, Japan). Three Reading Stations with Trained Retinal Readers. Low Lighting was Present in Room #2 Allowing for Maximally Dilated Pupils Without using a Mydriatic Agent.
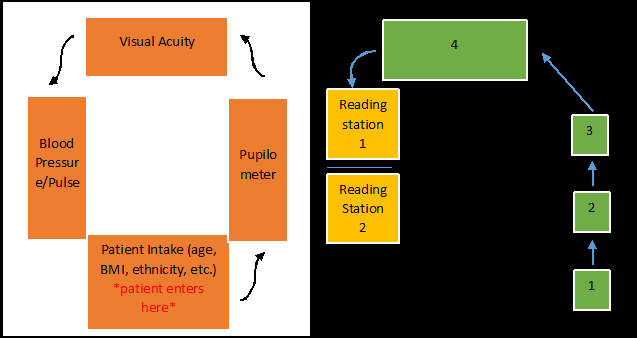
An IRB-approved screening protocol consisted of several stations (Figure 2 left room), included baseline data consisting of self-reported health, BMI, blood pressure, pulse, visual acuity (Snellen visual chart at 20 feet), and pupilometer. In room 2 (Figure 2 Right) a non-contact Canon automated puff tonometer7 with self-alignment head (Canon, TX 20 Tokyo, Japan) was used. It was not used on children under the age of 13, as we found it usually could frighten this younger population and limit their participation in the rest of the ocular wellness screening.
We used an auto-refractor (Canon, RKF2 Tokyo, Japan) that is computerized instrument to measure the individual’s refractive error. This specific model can also measure keratometry or the curvature of the cornea that deals with the level of astigmatism, a condition that may cause visual distortion when the cornea has an irregular shape. It should be noted that patients with a high glucose levels should not be measured as it could result in false reading.
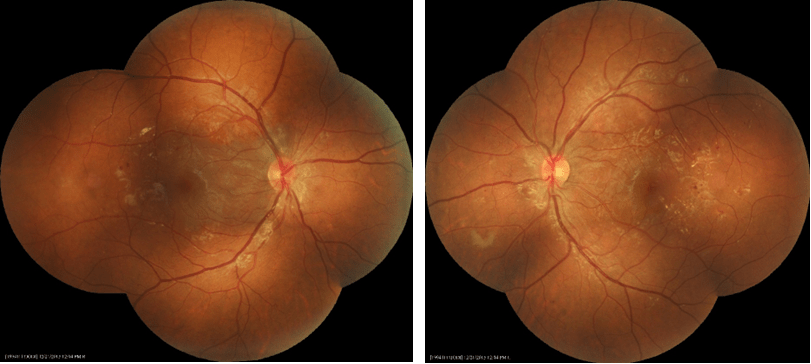
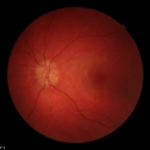
The next station was a 5 micron resolution Optical Coherence Tomography and Angiography (iVue and Avanti, Optovue, Fremont, CA, USA) and a Canon non mydriatic CR2 Plus-AF retinal camera with a digital EOS-60D camera back with a resolution of 24 Mp, (Canon, Tokyo, Japan) captures that can capture a 45-degree field of view of the posterior pole. Each participant had five images captured five images of their eyes: two anterior segment images (right and left) to uncover the presence of anterior segment opacities followed by two 45 degrees color images with a minimum pupillary dilation of 3.2 mm of the posterior pole of each eye, and one auto-fluorescence image of the posterior pole (usually left eye). Additional images could be captured in the event of proliferative diabetic retinopathy where wide angle views were needed up to a field of view of 90 degrees using a montage (Figure 3) module in the Canon Eye-Q software (Canon, Irvine, California, USA). The room light in the eye imaging room was dimmed to 125 candelas to limit reflections while capturing ocular images as well as to minimize the need to dilate the pupil before capturing ocular images. The specific order of instruments used in Room 2-Right (Figure 2) is that we perform all posterior pole scans and digital photography without the use of mydriatic agents to dilate the eyes.
Figure 3 (Right and Left). Color Images of the Posterior Pole Demonstrating A Field of View Equal to 90 Degrees (Set of 5 Retinal Photos Each) of A Participant with Diabetic Retinopathy
Captured digital images and data were reviewed by three (3) health care professional readers. The results were shared with screened participants and parent(s) (when appropriate) following the Health Insurance Portability and Accountability Act (HIPAA) privacy regulations. A copy of the posterior pole retinal imagery and OCT, OCTA scans were offered to all participants for their personal files. When on-site readers had a medical query, they could remotely connect from Florida to Rutgers Medical School in New Jersey, USA using DICOM and HIPAA protocols12 and have a board-certified ophthalmologist as seen in Figure 8 to review images and data and give a professional opinion to the on-site readers on the findings.
RESULTS
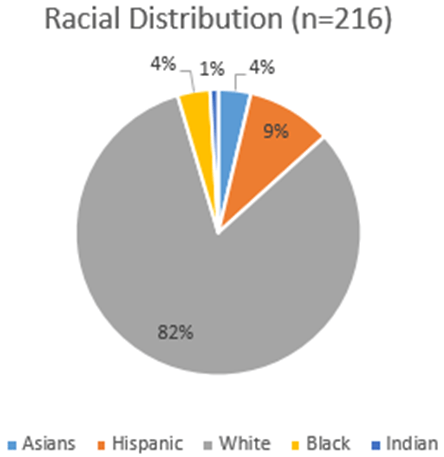
The average age in this cohort was 21-years-old (Table 1) with 76 males and 140 females. Eighty-two percent (82%) were White, 9% Hispanic 4% African American and 4% Asian (Figure 4). Average Body Mass Index (BMI) for this cohort was 21 that is considered healthy in the USA. The mean duration of T1DM was ten (10) years. With the introduction of the insulin pump,8 daily control in T1DM became easier allowing children to interact with non T1DM more seamlessly (average number of years using the IP was 7-years with 71% male and 79% female). Seventy five percent (75%) of our screened population had a Continuous Glucose Monitoring System (CGMS)9 (average number of years wearing this device was 3.7-years). Blood glucose levels in our cohort ranged between 69 and 411 mg/dL with a median blood glucose of 160 mg/dL (average self-reported 3-monthglycated hemoglobin (HbA1c) percentage was 7.1%. Average blood pressure was 123/71 for males and 118/72. Intra-ocular pressure was 18 mm of mercury in both eyes.
| Table 1: Summary of Screening at Friends for Life |
|
Male
|
Female
|
Number of Participants with Retinal Hemorrhages
|
|
76
|
140
|
|
| 2 – 12 years old |
23
|
40 |
3
|
| 13-19 years old |
21
|
43 |
14
|
| 20-30 years old |
19
|
34 |
10
|
| 30> years old |
13
|
23 |
14
|
| BG |
173
|
163 |
|
| A1C (Self-reported) |
7.45
|
7.05 |
|
| Having a CGM |
56 (74%)
|
105 (75%) |
|
| Having a pump |
54=71%
|
110=79%
|
|
| Artificial pancreas |
0
|
0
|
|
| BMI |
21.67
|
21.76
|
|
| BP |
123/71
|
118/72
|
|
| Pulse |
82
|
85
|
|
| IOP |
18 OU
|
18 OU
|
|
| 0-5 years of T1DM |
25
|
49 |
15
|
| 5-10 years of T1DM |
13
|
30 |
3
|
| 10-15 years of T1DM |
9
|
22 |
7
|
| 15+ years of T1DM |
14
|
21 |
13
|
Figure 4. Racial Distributions
Gold standard for diabetic retinopathy screenings remains color photography as described by the American Diabetes Association (ADA), American Academy of Ophthalmology (AAO), American Optometric Association (AOA) and Endocrinology. Consensus for these groups is for T1DM to undergo an ocular screening at 5-years post diagnosis or at the age of 15 years old.10 Of the 216 participants (432 eyes), our retinal color digital photography had positive findings in 41 participants totaling 82 dot hemorrhages, 15 flame hemorrhages, 3 IRMAs and 56 cata racts (Table 2). In our 10-year experience with screening this group of T1DM shows that a more sensitive diagnostic instruments such as fundus autofluorescence images (FAF)10 optical coherence tomography (OCT) and OCT-A (angiography) can reveal earlier posterior pole changes that can benefit from early intervention such as tighter blood glucose levels, limiting daily very high followed by very low blood glucose levels as well as good control of blood pressure.
| Table 2: Summary of Abnormalities from Retinal Images |
|
Number of Cases
|
% of Total Images with Abnormalities Found
|
| Dot hemorrhages |
82
|
5.4%
|
| Flame hemorrhages |
15
|
2%
|
| IRMAs |
3
|
0.5%
|
| Cataract |
56
|
13.6%
|
CASE REPORTS
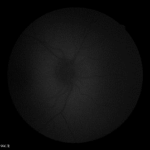
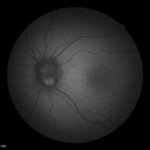
Three notable cases to best illustrate the importance of novel imaging technologies involve a 22- (Figure 5), 29- ( Figure 6) and 23-year-old (Figure 7) having T1DM for 16, 19 and 18-years, respectively, whom we have followed for 7-years.10,11 The 22-yearold was diagnosed with T1DM at age 6. Our initial encounter with her was when she was 16-years-old when she had T1DM for 10-years. When we performed color retinal imaging in her left eye, we noticed an irregular shape of the optic nerve head (ONH). After performing monochromatic auto-fluorescence of her ONH we picked up a strong positive (white) auto-fluorescence signal at 6 o’clock (Figure 5). Further color photography to include the nerve fiber layers (NFL) was performed to confirm if there was NFL drop-off or thinning. Participant had an IOP of 19 in both eyes and was sent back to the OCT for additional ONH scans in both eyes as well as NFL and GCC images. Although unrelated to T1DM, ONH drusens can affect NFL as well as the CGC resulting in peripheral loss of vision in a similar fashion as glaucoma. We continue to monitor her T1DM as well as possible complications due to the ONH drusen including visual fields.
Figure 5: Summary of data from 22 year old female with optic nerve head drusens in both eyes. Note color photo of the right eye shows no evidence of ONH drusen however, OCT clearly indicates ONH drusen in both right and left but larger in left eye.
| |
Right Eye
|
Left Eye
|
Left Eye Progressive Scan, OCT / OCTA 2015 to 2018
|
| OCT |
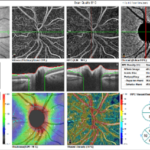 |
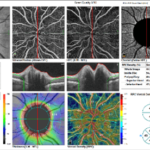
Note NFL drop off in superior quadrant |
 |
| Retinal Images |
|
|
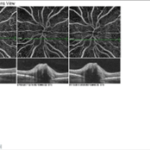
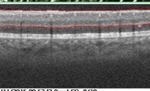
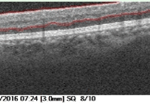
Figure 6. Summary of Data from 29 Year Old Subject with Progressive Fluid Buildup (Seen In Oct – A Images)
|
|
July 2015 |
July 2016
|
July 2017
|
| Glucose (mg/dL) |
74
|
180
|
160
|
| BP (mmHg) |
119/65
|
136/82
|
127/69
|
| IOP |
N/A
|
OD: 25
|
OD:22
|
|
OS: 22
|
OS: 23
|
| OCT |
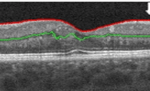 |
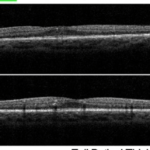 |
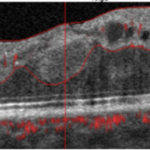 |
| OCT A |
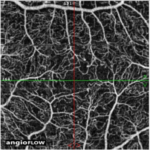 |
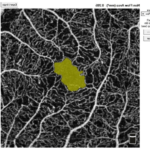 |
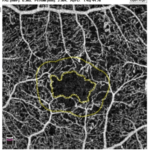 |
| Retinal Images |
N/A
|
 |
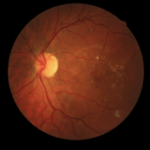 |
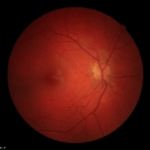
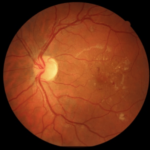
Figure 7. Summary of Data from 23 Year Old Subject with Improving Retinal Damage (Seen in Retinal Images)
| |
July 2015
|
July 2016
|
July 2017
|
| Glucose (mg/dL) |
319
|
204 |
288
|
| BP (mmHg) |
142/62
|
116/68 |
126/80
|
| IOP |
N/A
|
OD: 17
|
OD: 19
|
|
OS: 17
|
OD: 18
|
| OCT |
 |
 |
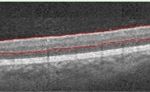 |
| OCT A |
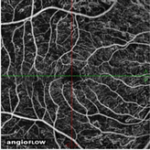 |
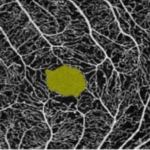 |
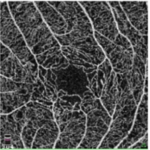 |
| Retinal Images |
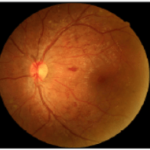 |
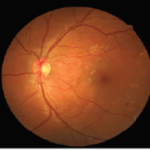 |
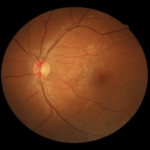 |
For the 29-year-old individual, 3-years ago his blood glucose levels were under control. His color retinal images show only a few hemorrhages (Figure 6). Although this individual had access to a continuous glucose monitor, his glucose control was poorly controlled and HbA1c level rose steadily to over 9%. He has had T1D for 19-years and used an insulin pump for nine years and a CGMS for 1.5-years. His glucose level at the time of screening was 212 and his HbA1c level was 7.8%. His intraocular pressure was 22 and 23 (OD, OS). At the time of his visit in July, his color retinal images showed hemorrhages and in the OCT analysis, there was evidence of significant macular edema. In addition, his foveal avascular zone using the OCTA was abnormal. Upon consultation at the screening event, he started to harness his glucose level and return to a safer range. He was observed again in August 2017. At this time, he showed slight improvement. We noted a small amount of edematous tissue in the macular area especially in the left eye when looking at the OCT and it is hoped that if he continues with a tighter control his glucose. He was referred for clinical follow-up.
The 23-year-old individual was diagnosed with diabetes in 1999. His first screening at Friends for Life was in 2010, when he was 15-years-old. His retinal images showed good control of diabetes. His blood glucose was 184 mg/dL, blood pressure was 128/78. After returning in 2013, there were early signs of retinopathy but no severe changes were noted. His self-reported blood glucose was 421 mg/dL, self-reported HbA1c level was >13%, blood pressure was 130/91 mmHg, and pulse 106 bpm. Retinal findings included 2 small dot hemorrhages, no flame hemorrhages, and a single intraretinal microvascular abnormality (IRMA). He received counseling regarding tight control of his diabetes.
At his 2015 screening, the participant had negatively changed his lifestyle and had poor control of his glucose. He noted that he felt numbness and pain in his legs which are associated with peripheral diabetic neuropathy. His blood pressure was 142/62 mmHg, fasting blood glucose was 319 mg/dL, and HbA1c level was >13.5%. Retinal changes were severe with a total of 440 dot and flame hemorrhages in both the eyes. The hemorrhages were localized within the posterior pole characteristic which is characteristic of diabetic retinopathy. The OCT-A images showed signs of retinal telangiectasia and occasional microaneurysm. All of these findings led to the conclusion that the patient had developed proliferative diabetic retinopathy. However, OCT-A findings, which indicated an avascular foveal pit, suggested immediate behavioral changes was required and monthly clinical follow-up was needed to prevent disease progression.
Over the course of the next year, the subject revised his approach to diabetes and made positive life changes. When he came back for the screening in 2016, his HbA1c had regressed to 8.1%, blood pressure was noted as 110/75 mmHg, and most hemorrhages had resolved leaving only two dot hemorrhages, no flame hemorrhages, and no IRMAs. In 2017, he continued to have good control of his diabetes. His HbA1c was 7.2%, blood pressure was measured as 124/70 and seven dot hemorrhages were found. This case demonstrates the value of early detection, follow-up and the importance of good glycemic and blood pressure control, (Figure 7).
CONCLUSIONS
Technological advances such as CGMS and pumps have allowed better control of blood glucose levels and avoid some of the challenges in daily life for individuals with T1DM. In our 10-year experience with screening T1DM is that a more sensitive diagnostic instruments such non-mydriatic retinal cameras with high resolution sensors (over 24 Mp of resolution) equipped with fundus autofluorescence capabilities (FAF)10 is key in producing useful clinical data. Optical coherence tomography (OCT) and OCT-A (angiography) can reveal earlier posterior pole changes such as the images produced by the OCT-A for the foveal avascular zone (FAZ) as illustrated in our case presentation. We feel that a base-line imaging session could be a useful standard to include a 45 degree field of view retinal imaging at the time of diagnosis in addition to OCTA with a view of FAZ, ganglion cell complex (GCC) seen by the OCT as well as confirming the absence of macular edema using the OCT. Early detection is key for early intervention and maintaining a healthy eye. Since we share the images with T1DM individuals or care takers (if child is bellow 18), they can see any changes in the posterior pole of the eye (retina) and understand the benefit from early intervention and a tighter blood glucose levels, limiting daily very high followed by very low blood glucose levels as well as good control of blood pressure.
We cannot emphasis enough the benefits of weeklong gathering such as ‘Friends for Life’ (www.childrenwithdiabetes.com) events where emphases are placed on T1DM education for family members, friends and children affected with T1D helps individuals that are going through the same process of daily management of their diabetes. They have many opportunities to exchange tips and life experiences, fears and most importantly success stories connected through social media. These relations also help the children get into a daily routine that will ensure proper and good control of T1DM.
ACKNOWLEDGEMENTS
CWD 2017 team: Rob Freund, RN, Sarah Melendez, RN, Kimball Dunlap, RN, Belinda Peach, Stephanie Lambert, Paula Fairchild, Doris Chong, D. Smith, B. Schmidt, J. Lopez, Donna Cope, Scott Kyllo, Laura Billetdeaux, and Jeff Hitchcock.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.

































