INTRODUCTION
Upper tract urothelial carcinoma (UTUC) includes any malignant tumors derived from the urothelium between the level of the renal pelvis and the distal ureter.
The vast majority of tumors from the upper tract are of urothelial origin (>90%). They can present as carcinoma in situ (CIS), as papillary or sessile lesions, and as solitary lesions or in a multifocal pattern.1
Urothelial carcinomas (UC) appear in the renal pelvis, ureter, or urethra much less frequently than in the bladder. In 2004, the World Health Organization (WHO) added histological variants of UC, which included lymphoepitheliomatoid cells, sarcomatoid, plasmacytoid, microcystic, micropapillary, nested, and small cell, each with different biological behaviors.
The nested variant urothelial carcinoma (NVUC) is an uncommon histological type described as an invasive form of UC,2,3 with an estimated incidence of 0.3%.4 It is characterized by a nested pattern, with a low-grade histological appearance with aggressive biological behavior,5,6 islets of atypical, soft urothelial cells that strongly simulate von Brunn’s nests and invade the lamina propria or deeper.2,3
CLINICAL CASE
A clinical case of a 70-year-old man with a medical history of hypertension and a former tobacco user is presented.
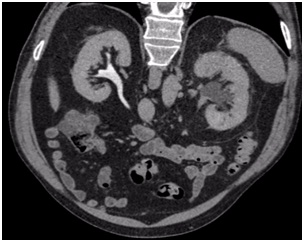
He was admitted to hospital with colic-type left lumbar pain associated with a single episode of gross hematuria without clots. He had experienced a similar clinical episode 2-months before which was self-limiting, so he did not consult. Urine analysis showed microhematuria. Abdominal ultrasound reported a 16 mm diameter left pelvicalyceal dilation. Abdomen and pelvis computerized tomography (CT) scan showed an enlarged left kidney associated with thickening of the anterior and posterior pararenal fascia and pelvicalyceal dilatation associated with a non-calcified soft tissue density in the region of the pelvis and the left proximal ureter (Figure 1). A contrast enhanced study confirmed the presence of an endoluminal filling defect with a soft tissue density and marked delay of contrast drainage from the left kidney (Figure 2).
Figure 1. Unenhanced CT Scan
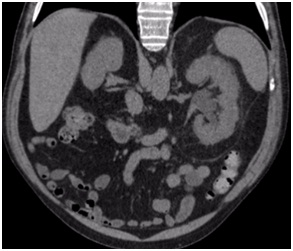
Figure 2. Enhanced CT Scan
Due to the acute presentation and intense pain, cystoscopy and left ascending pyelography were performed, which demonstrated a 4 cm uretero-pyelicstricture, moderate pelvicalyceal, and marked delay in contrast evacuation. Ureteroscopy revealed the strictured segment with inability to progress a 6 and 4.8 French ureteral catheters. Cytology and distal biopsy of the stenosed area was taken with negative results for atypia. Left percutaneous nephrostomy and nephroscopy were performed with no apparent evidence of injury, but without being able to cross the ureteropelvic junction stricture.
Two weeks later, an exploratory laparoscopy was performed, which showed uretero-pelvic junction with inflammatory characteristics,. The decision was made to perform a segmental resection of the proximal ureter. Frozen section were taken but the pathology report was not definitive.
Due to the extensive involvement of the ureter, renal pelvis, the difficulty of subsequent reconstruction and, eventually, the high clinical suspicion of a neoplastic process, a left nephroureterectomy with a laparoscopic bladder cuff was performed.
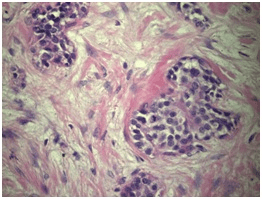
The definitive pathological report was invasive urothelial carcinoma with characteristics compatible with a nested variant (nested), located in the renal pelvis with complete invasion of the wall, including the muscularis and peri-pelvic adipose tissue. The final pathology report showed focal tumor infiltration of the ureteral segment with a distal margin free of tumor involvement. The renal parenchyma was tumor-free, pathological staging was pT3; Nx (Figures 3 and 4).
Figure 3. H&E 40xPanoramic
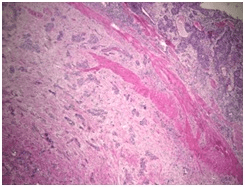
Figure 4. H&E 400x
One-month later, laparoscopic left retroperitoneal lymphadenectomy was performed. Thirteen lymph nodes were removed with a negative result.
Adjuvant chemotherapy (AC) was performed with cisplatin and gemcitabine due to the high risk nature of the cancer, with a good clinical response.
DISCUSSION AND CONCLUSION
Nested variant urothelial carcinoma is an atypical histological variant with a high potential of malignancy. The vast majority of reported cases refer to their being found in the bladder.1,7 Approximately 50% are around ureteric orifices.1,7,8
Despite its low-grade appearance in morphology, NVUC appears to behave like a high-grade tumor, at least in the bladder.2,3 The World Health Organization (WHO) has reported that 70% of patients died 4 to 40-months after diagnosis, despite treatment.2 According to microscopic characteristics, NVUC nests may show a microcystic or cribriform pattern and rarely small tubules.8,9 Mild anisonucleosis and relatively soft chromatin are evident with a tendency to increase atypia with increasing invasion at depth. The cytological characteristics of the nested variant are subtle and can be confused with reactive changes, and are therefore lost in urine cytology.10 Furthermore, the preference of this variant of submucosal growth over exophytic growth may be the reason for the absence of urinary signs and symptoms that lead to a possible delay in diagnosis.
Differential diagnoses that can be made are benign, such as Von Brunn’s nests, glandular cystitis, cystitis cystica, and malignant, such as carcinoid or paraganglioma.8,11
In their review, Veskimäe et al reported similar oncological outcomes after radical cystectomy in patients with nested variant compared with pure urothelial carcinoma in bladder.12
According to European Association of Urology (EAU) 2020 guidelines, the case reported was a high risk UTUC and radical nephroureterectomy with bladder cuff is the standard treatment for those cases, regardless the tumor location.13 In high risk cases, retroperitoneal lymphadenectomy improves the cancer specific survival and reduces the risk of local recurrence14 even in clinically15 and pathologically16 node negative patients
Recently Birtle et al published the results of the peri-operative chemotherapy vs. surveillance in upper tract urothelial cancer (POUT) trial, a phase 3, randomized clinical trial comparing platinum-based peri-operative adjuvant chemotherapy vs. surveillance in high-risk UTUC patients. The results showed a statistically significant benefit in favor of chemotherapy, with a 55% decrease in the relative risk of disease recurrence or death (HR 0.45, 95% CI 0.30-0.68; log-rank p=0.0001). However, in the absence of evidence based treatment for with patients with NVUC alone.4,17,18 radical surgery remains the treatment of choice.19
Seisen et al published an observational study of 3,253 patients diagnosed with UTUC, T3/T4 N0, or N+ staging who underwent radical nephroureterectomy, and subsequently studied the follow-up of patients who underwent adjuvant chemotherapy and those who were observed. They showed a greater overall survival for the AC group over the observation (47.41-months vs. 35.78-months; p>0.001).20
Controlled clinical trials evaluating the role of chemotherapy in UTUC with histological variants are needed.
To conclude, NVUC is a histologically unique tumor, which should not be confused with other tumours, due to its poor prognosis. Currently, the action protocol for this specific diagnosis is not standardized given its low incidence, but in principle, radical surgery would be indicated with patient, clinical, imaging and endoscopic follow-up.
CONSENT
The authors have received written informed consent from the
patient.
FINANCIAL DISCLOSURE
There are no funding sources.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









