INTRODUCTION
Mucormycosis disease or informally known as ‘black fungus’ is the fungal infection observed in patients who have recovered from coronavirus disease-2019 (COVID-19). Yellow fungus and white fungus are the other fungal infections associated. This non-contagious fungal infection begins its infection with invasion of the sinus followed by diffusion into the intracranial and infraorbital regions which is responsible for blackened or discoloured nose, chest pain, coughing blood, double or indistinct vision, and difficulty in breathing. The causative organism for this fungal infection is the mucor mold (mucormycetes) usually found in air, soil, and also in the mucus and nose of human beings.1
A mould fungi of the order Mucorales, class Zygomycetes and genus Mucor, Rhizomucor, Rhizopus, Saksenaea, Cunninghamella and Absidia cause an angioinvasive ailment, Mucormycosis.2,3 An infection with high-levels of fatality is caused by Rhizopus oryzae, accountable for nearly 60% of mucormycosis and 90% of rhino-orbital-cerebral mucormycosis (ROCM) form.4 In 1855, Friedrich Küchenmeister elaborated the first case of the black microscopic enemy and in 1876, Fürbringer firstly explained the occurrence of this disease in lungs.5
Recent reports indicate a steep rise in the number of mucormycosis cases worldwide and particularly in India. The incidence of the fungal disease is generally observed in COVID-19 recovered patients, who had risk factors like diabetes and steroid induced high glucose levels, hypoxia, diabetic ketoacidosis (DKA), acidic medium due to metabolic acidosis, immunosuppression leading to decreased white blood cells (WBC) phagocytic activity and increased ferritin. Other reasons include wiping out of the potentially morbific bacteria along with their protective commensals owing to the use of broad-spectrum antibiotics. Sometimes the use of Voriconazole, an antifungal drug, prevents only Aspergillosis and the mucor remains intact and flourishes due to dearth of competition. Ventilation for a longer term also decreases immunity and it is speculated that the fungal transmission takes place with the humidifier water given with oxygen.6
An evaluation in the year 2019-2020 established that, the population worldwide ailed by mould disease ranged from 0.005 to 1.7 per million, while in India, its occurrence was almost 80 times higher (0.14 per 1000) in comparison to developed countries.7,8 In India, the most common risk factor associated with zygomycosis is diabetes mellitus, while in USA and Europe, the common risk factor is organ transplant and hematological malignancies. Generally, the long-term use of corticosteroids are the cause for several devious/opportunistic fungal infections including mucormycosis and aspergillosis but recent studies reveal that corticosteroids used even for a short course causes mucormycosis in people having comorbidities like diabetes.8,9,10
The unprecedented findings above need a reconsideration in the COVID-19 context where corticosteroids are used vehemently. It is an outcome of immense importance especially in terms of public health as the mortality rate is quite high in this fungal disease. It is as high as 90% in case of the mucormycosis with intracranial involvement.11 Still another matter of concern is the rapid propagation of the disease such that a half day delay in diagnosis could be deadly and this sums up to the cause of diagnosis of more than 50% of the mucormycosis only during the post-mortem autopsy series.12
CLINICAL MANIFESTATIONS OF MUCORMYCOSIS
Mucormycosis invades the heart, lungs, kidney, central nervous system, skin, gastrointestinal tract, sinuses, jaw bones, joints, nose, orbit and mediastinum (invasive type), although in clinical practice, ROCM is the most common form observed all over the world (Table 1).
| Table 1. Clinical features of Mucormycosis |
| Sr. No. |
Types of
Mucormycosis |
Risk Factors |
Clinical Features |
| 1 |
Pulmonary |
Neutropenia,13 bone marrow and organ transplant, and hematological malignancies |
Fever, chest pain, cough, shortness of breath |
| 2 |
Rhino-orbito-cerebral |
Diabetes |
Facial oedema, proptosis, diplopia, palatal or palpebral fistula, strokes, hemorrhages. |
| 3 |
Gastrointestinal |
Neonates |
Fever bleeding per anus, mass like lesions, intestinal perforation |
| 4 |
Cutaneous and soft-tissue |
Immunocompetent patients, traumatic injury |
Abscesses, erythema, induration, necrosis, dry ulcers, and eschars |
| 5 |
Bones and joints |
|
Pain, tenderness, cellulitis, |
The fungal spores known as the sporangiospores enters the human body through inhalation causing pulmonary infection. This infection occurs mainly in patients with organ and bone marrow transplant, neutropenia13 and malignancies at hematological level. It spreads closely into the abdominal organs through the diaphragm. The clinical features incident in pulmonary mucormycosis are analogous to COVID-19 with headache, fever, shortness of breath, cough, altered mental status thus making clinical diagnosis tough. The fungal infection can be suspected when a patient under appropriate medications is not getting well and is rather showing inexplicable deterioration.14 Radiological conclusions often vary in size, configuration, distribution and number of lesions.
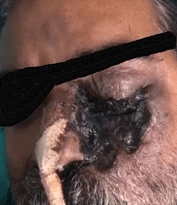
ROCM typically develops in diabetes and haematology patients but then they seldom develop lung infections.13,15 The origin of this infection is usually from the paranasal sinuses causing destruction of bone and subsequently invading the eye, orbit and brain (Figure 1).16,17,18,19 Consequently, it leads to proptosis, palpebral fistula, and one-sided facial oedema. Invasion of brain due to blood vessel blockage can cause strokes and may be fatal too. Other possible symptoms include drowsiness, headaches, limb weakness, and seizures.
Figure 1. Cuteneous and Rhino-Orbito-Cerebral Mucormycosis (Showing Involvement of Skin, Sinus and Eye)
The rarest manifestation of black fungus is the gastrointestinal disease with symptoms almost the same as that of common gastrointestinal diseases but these manifestations are much common in neonates with a high death rate.
Immunocompetent patients are hit by rather common fungal infection, soft-tissue and cutaneous mucormycosis. It occurs after skin disruption owed to traumatic injuries (motor vehicle accidents, natural disasters, or iatrogenic sources), burns or surgery.20,21 The characteristic symptoms are abscesses, dry ulcers, skin swelling, necrosis, and eschars.
The pathological trademark of mucormycosis is giant cell invasion, underlying tissues eosinophilic necrosis and thrombosis.
MORTALITY
Mortality rates for this epidemic disease ranges as high as 40% to 80% and is dependent on sites of infection and underlying conditions.13 The worst scenario is observed in extensively burnt patients,22 hematopoietic stem cell transplantation (HSCT) recipient patients and one with haematological malignancies.13 The mortality rate in case of disease dissemination to the central nervous system (CNS) is higher than 80% whereas skin infection or localised sinus is associated with low mortality rates. The immunocompromised patients and neonates with gastrointestinal mucormycosis also have high mortality rates probably due to polymicrobial sepsis and delay in diagnosis. In order to improve the survival rates, there must be an early and prompt diagnosis followed by timely multidisciplinary treatment methods involving antagonistic surgical debridement.23
DIAGNOSIS
The diagnosis of the fungal infection depends on the sound imaging techniques, trained personnel’s, and histological and mycological investigations.24 Summary of diagnostic methods are given in Table 2.
| Table 2. Diagnostic Methods for Mucormycosis |
| Sr. No. |
Investigations |
Findings |
| 1 |
Blood |
ESR, CBC, HbA1C, FBS, PPBS, KFT, LFT with electrolytes, Viral markers (HCV/HIV/HBV) |
| 2 |
Nasal endoscopy |
Scabbing, crusting, discoloured mucosa, granulation |
| 3 |
Radiological |
CECT chest, Nose and PNS
CEMRI Brain Orbit and Face |
| 4 |
KOH staining and microscopy |
Non-septate/pauci-septate |
| 5 |
Histopathology |
Infarction, coagulation necrosis, angioinvasion, infiltration by neutrophils |
| 6 |
Culture |
Cotton white or greyish black colony |
Suspected pulmonary and haematological malignancy mucormycosis patients are recommended to undergo pulmonary computerized tomography (CT) scan for the reversed halo sign detection, vessel occlusion on CT pulmonary angiography, or a ground glass opacity area bounded by a consolidation ring on thoracic CT.
Diabetic patients with sinusitis, facial pain, ophthalmoplegia or proptosis are recommended to undergo magnetic resonance imaging (MRI) or cranial CT scan for diagnosis of the disease. Endoscopy is strongly endorsed to detect mucormycosis in case sinusitis is diagnosed in the above situation.
If disease of brain or eye is conjectured, then MRI is recommended in place of CT scan due to significantly higher sensitivity. Biopsy is strongly suggested in case of potential prognosis of mucormycosis.
A patient with primary malignancy on contracting mucormycosis is recommended to undergo thoracic, abdominal and cranial imaging studies to identify the level of the disease.
The clinical diagnosis of mucormycosis is based on Smith and Krichner (1950)25 criteria which are still contemplated to be benchmark and include:
(i) Black, necrotic Turbinate’s turned black and undergone necrosis to be easily flawed for dried and crusted blood,
(ii) Facial pain and nasal discharge of blood-tinge, both on one side,
(iii) Peri-nasal swelling or soft peri-orbital with induration and discoloration,
(iv) Blepharoptosis of the eyelid, proptosis or exophthalmos of the eyeball and complete ophthalmoplegia of eye muscles and,
(v) Numerous cranial nerve palsies incongruent to documented lesions.
HISTOPATHOLOGY IN MUCORMYCOSIS
The clinical samples are histologically examined for diagnosis of mucormycosis wherein specimens stained with blankophor (Tanatax Chemicals, Ede, The Netherlands) or calcofluor white, fluorescent brighteners (Sigma Aldrich, St Louis, MO, USA) are subjected to direct microscopy. The non-pigmented hyphae shown in the tissue sections stained with periodic acid-schiff stain (PAS), Grocott-Gomori’s methenamine-silver stain (GMS) or haematoxylin-eosin (HE) helps in conformation of infection.26
Histopathological studies reveal that the hyphae are 6-16 µm in width and may be pauci-septate, sparsely septate or non-septate. It has ribbon-like appearance with an irregular branching pattern.26 In acute lesions, coagulation necrosis, haemorrhagic infarction, perineural invasion, vascular invasion, neutrophil infiltration (in non-neutropenic hosts) are distinctive features27; while, in chronic lesions, a chronic pyogranulomatous inflammation containing giant cells, and sometimes splendore-hoeppli phenomenon causing enclosure of hyphae by the asteroid bodies (deeply eosinophilic material),28,29 are realized.27,30
Immunohistochemistry utilizes polymerase chain reaction (PCR) techniques or commercially available monoclonal/polyclonal antibodies31,32 on either fresh or formalin-fixed paraffin-embedded tissue which is highly specific for detection of specific antigens.
For testing the antifungal susceptibility and identifying their genus and species, culture of fungal specimens is strongly recommended. The tissue should be incubated at 30 °C and 37 °C separately. Clinical specimens are subjected to direct microscopy using fluorescent brighteners with main focus on hyphal width, septation, and branching angle.
TREATMENT FOR MUCORMYCOSIS
The treatment should be taken up immediately after the diagnosis of mucormycosis is established.33,34,35 The effectiveness of the treatment depends on the availability of antifungal drugs and appropriate surgical techniques. Here we are summarizing treatment of mucormycosis in Table 3.
| Table 3. Management of mucormycosis34,35,36,37,38 |
| 1 |
Control of diabetes |
| 2 |
Reduce steroids |
| 3 |
Discontinue other immunomodulating drugs like:
Baricitinib, Tofacitinib |
| 4 |
Surgical debridement
a. Endoscopic sinus surgery debridement: when only sinus and nasal involvement.
b. Maxillectomy: when maxilla bone is involved.
c. Maxillectomy and debridement of orbit and zygomatic bones.
d. Exenteration of eye: when total ophthalmoplegia, loss of vision, orbital tissue necrosis and chemosis is present.
e. Frontal bone and skull base: when skull bone and cerebral parenchyma are involved. |
| 5 |
Medical Treatment
a. Maintain adequate systemic hydration, infuse normal saline IV before amphotericin B infusion
b. Antifungal therapy
i. Liposomal amphotericin B (1-10 mg/kg/day)
ii. Amphotericin B deoxycholate (1.0-1.5 mg/kg/day)
iii. Amphotericin B lipid complex (5 mg/kg per day)
iv. Posaconazole (200 mg dose four times per day) or Isavuconazole (injection/tablets) |
| 6 |
Prevention of ROCM
In hospital:
a. Maintain cleanliness and oral hygiene with Iodopovidone gargles or mouthwashes.
b. While oxygen administration, the humidifier must be leakage free and sterile water must be used for humidification.
c. Administering steroid must be limited and need based with strict control over blood glucose levels.
d. Avoid the use of antifungals and broad-spectrum antibiotics as it destroys the normal commensal flora ensuing growth of unwanted organisms basically due to lack of competition.
After discharge from hospital:
a. Stay indoors stay safe.
b. Do regular exercises.
c. Control blood sugar levels.
d. The surroundings must be hygienic and devoid of dampness and dust.
e. Continue maintaining nasal and oral hygiene.
f. Always wear N-95 mask while moving out.
g. Keep away from fields, construction areas, and grounds.
h. Plants and the soil in which they grow are the areas where the fungi proliferate. Hence better avoid gardening or working with soil. Under unavoidable circumstances, use rubber gloves, masks, and boots. |
For minor infections, oral antifungal medications suffice but, for most invasive cases injections are to be administered. These drugs are capable of targeting the fungal varieties in the body and slow down the rate of infection spreading in the system, and completely restrain their destructive activity.
The key factor that increases the effectiveness of mucormycosis treatment is an early, immediate and accurate diagnosis followed by provision of urgent medical care by a medical team of specialists which includes internal medicine experts, microbiologist, ears, nose and throat (ENT) specialist, neurologist, ophthalmologist, plastic surgeon, dentist in maxillofacial procedures, and biochemist. Consequently, there is reduced damage in body organs, thwarts mycological infection totally, thereby preventing severe complications and fatal outcomes. These patients with diabetes are advised to undergo regular blood glucose level examination and in case of sudden rise in sugar level in blood, seek medical care at the earliest. In cases of severe mutilation of bodily tissues by the fungus, surgical procedures are taken up to get rid of these mycetomas or fungal balls. The remedial measures might heal the patient to some extent but the physician must keep a constant check on the patient to avoid recurrence of fungal infection and thus assuring complete recovery of the patient.
ANTIFUNGAL DRUGS
1. 1.0 -1.5 mg/kg/day of Amphotericin B injection.34
2. 1- 10 mg/kg/day of Liposomal amphotericin B injection36– In numerous cases, this injection treated mucormycosis successfully with different patterns of organ involvement. In case of CNS involvement liposomal amphotericin B at a dosage of 10 mg/kg per day should be used.
3. Injection Amphotericin B lipid complex- If the CNS involvement is absent then ABLC can be administered in dosage of 5 mg/kg per day. Although the kidney transplant recipients are given 10 mg/kg per day doses of this injection.
4. AZOLE Derivatives – Posaconazole is broad-spectrum antifungal triazole available in both oral and parenteral formulations. It is given as 200 mg dose four times per day. Alternatively, posaconazole delayed-release tablets is taken with food as a dose of 300 mg twice on first day and then 300 mg once daily.
Treatment can extend from 14 to 21-days depending on rebuilding of the host immune system and resolution of primarily symptomatic findings on imaging.
Though the efficacy of amphotericin B and isavuconazole formulations are similar, isavuconazole shows less hepatotoxicity but can shorten the QTc interval in contrast to other mould active drugs.
The first-line treatment for all organ involvement patterns is use of 5-10 mg/kg per day liposomal amphotericin B. This dosage can be reduced in cases of development of substantial renal toxicity.
First-line antifungal combination therapy- Some antifungal combinations therapy had been found to improvise on the survival and cure rates with no dissension observed in animal models.34,37,38
SURGICAL TREATMENT FOR MUCORMYCOSIS
Along with systemic antifungal treatment, early comprehensive surgical treatment is strongly supported by the guideline group.34 Debridement or resection should be repeated as per the requirement. Following procedures are charted as per the involvement of area and bone erosion:
1. Endoscopic sinus surgery debridement: when only sinus and nasal involvement is there, without bone erosion.
2. Maxillectomy: when maxilla bone is involved.
3. Maxillectomy and debridement of orbit and zygomatic bones.
4. Exenteration of eye: when total ophthalmoplegia, loss of vision, orbital tissue necrosis and chemosis is present.
5. Frontal bone and skull base: when skull bone and cerebral parenchyma are involved.
PROPHYLAXIS RECOMMENDATIONS
Primary prophylaxis for patients with neutropenia or with graft versus host disease (GvHD) is administration of posaconazole delayed release tablets with moderate strength, and administration of oral suspension with marginal strength to prevent mucormycosis.34
Secondary prophylaxis – In immunosuppressed patients having previous diagnostic history of mucormycosis, is strongly recommended surgical resection and prolongation of the last drug effective to the patients.
PREVENTION Of ROCM
In Hospital
1. Maintain cleanliness and oral hygiene with Iodopovidone gargles or mouthwashes.
2. While oxygen administration, the humidifier must be leakage free and sterile water must be used for humidification.
3. Administering steroid must be limited and need based with strict control over blood glucose levels.
4. Avoid the use of antifungals and broad-spectrum antibiotics as it destroys the normal commensal flora ensuing growth of unwanted organisms basically due to lack of competition.
After Discharge from Hospital
5. Stay indoors stay safe
6. Do regular exercises
7. Control blood sugar levels.
8. The surroundings must be hygienic and devoid of dampness and dust.
9. Continue maintaining nasal and oral hygiene.
10. Always wear N-95 mask while moving out.
11. Keep away from fields, construction areas, and grounds.
12. Plants and the soil in which they grow are the areas where the fungi proliferate. Hence better avoid gardening or working with soil. Under unavoidable circumstances, use rubber gloves, masks, and boots.
A patient undergoing post-COVID recovery, if develops eye pain, sinus headache, toothache, facial pain, headache, stuffy nose, seizures, bloody nasal discharge, limb weakness, blackish discoloration over palate or nose, swelling, diminished vision or double vision, or drowsiness, then immediate medical expert advise must be attained.
CONCLUSION
The mucormycosis increased in India appears to be due to the high incidence of diabetes, use of corticosteroid which increases blood glucose levels, and COVID-19 that causes lymphopenia, cytokine storm, and endothelial damage sets a site for opportunistic fungal infection. Adequate control of hyperglycemia and the use of corticosteroids in an evidence-based and judicious manner in COVID-19 patients is strongly recommended to reduce the burden of fatal mucormycosis. Early complete surgical treatment of mucormycosis in addition to systemic antifungal treatment could result in a better survival rate of patients.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
The authors have received written informed consent from the patient.






