CASE HISTORY
A 58 year old, G1P0010, presented with complaints of abdominal pain and rapid weight loss. A large, smooth mass was palpated 2 cm above the umbilicus. CA-125 and CEA were found to be elevated at 55.6 U/mL and 20.1 U/mL, respectively. Physical examination suggested a large, heterogeneously enhancing pelvic mass extending into the lower abdomen with cystic and solid features. The patient denied family history of gynecologic cancers.
An exploratory laparotomy including a total abdominal hysterectomy, bilateral salpingo-ophorectomy, and optimal tumor cytoreductive surgery was conducted. A pelvic mass, approximately 20 cm in size, was excised from 3-4 cm above the umbilicus. The mass was smooth and immobile, originating from the left adnexa with multiple cystic structures.
Two histologically and immunohistochemically distinct cancers were identified. One, a mixed epithelial tumor accounting for approximately 70 percent of the mass; the other a germ cell tumor positive for malignant-high grade carcinoma. The mixed clear cell carcinoma and yolk sac tumor were present in the left ovary with a single metastatic nodule to the sigmoid mesentery and positive washings. Final staging was consistent with International Federation of Gynecology and Obstetrics (FIGO) Stage IIB (IIC by old staging criteria) ovarian carcinoma. Colleagues at the Brigham and Women’s Hospital confirmed the pathologic diagnosis.
The case was presented at a multidisciplinary committee meeting. Gynecologic oncology colleagues at Magee Women’s Hospital and Hahnemann University were also consulted in light of the rare tumor histology. A decision was made to use the chemotherapeutic regimens associated with CCC and YST treatment to aggressively address the patient’s cancer. Extrapolation from current literature produced the plan to start the patient on 4 cycles of intravenous bleomycin/etoposide/cisplatin (BEP), followed by 4 cycles of Carboplatin/Paclitaxel.
The BEP regimen for this patient was Etoposide 100 mg/m2 (159 mg), Cisplatin 20 mg/m2 (32 mg), and Bleomycin 30 units. Carboplatin/Paclitaxel was administered every 21 days. The Paclitaxel was given at a dose of 175 mg/m2 (273 mg for this patient) and Carboplatin AUC of 6 mg (460 mg for this patient). The patient completed 3 cycles of BEP and suffered blisters on her hands and feet, presumably due to Bleomycin. Rather than foregoing the final cycle of BEP, the patient’s fourth cycle of chemotherapy excluded Bleomycin. She completed 2 cycles of Carboplatin/Paclitaxel chemotherapy which were subsequently discontinued secondary to progressive delays due thrombocytopenia. AFP levels have fallen from 106 ng/Ml 1 month post-operatively to 1.4 ng/mL approximately 7 months after surgery. Her CA-125 levels fell to 4.1 U/mL 7 months after surgery. Both CA-125, which was initially elevated, and AFP were used to follow the treatment response. On recent follow up one year after surgery the patient’s CA-125 and AFP reference readings were 4.1 and 1.4, respectively.
PATHOLOGIC FINDINGS
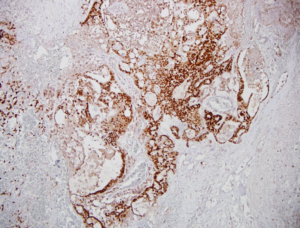
Pathologic investigation identified two distinct histologic and immunohistochemical patterns consistent with a mixed epithelial, accounting for roughly 70% of the tumor, and germ cell tumor. Gross examination of the left ovary demonstrates a 22.5 × 15.0 × 8.0 cm and 1020 gram tumor with a dull, pink-tan, and smooth, intact surface. The cut section shows predominantly solid and necrotic areas with cystic spaces filled by hemorrhagic, gelatinous debris. The right ovary, uterus, fallopian tubes and omentum were free of tumor. The H & E microscopic sections from the ovarian tumor and from the mesenteric nodule demonstrate a tumor with biphasic morphology and immunophenotype. There were areas of clear cell carcinoma (Figures 1A and 1B) and areas of yolk sac tumor with glandular differentiation and Schiller-Duval bodies (Figure 2A). A distinct immunohistochemical profile characteristic of each tumor was also evident (Figures 2B and 2C). The clear cell carcinoma component is positive for PAX-8, EMA, and HNF-1β, and negative for SALL-4 and Glypican-3. On the other hand, the yolk sac tumor demonstrates reactivity with SALL-4 and Glypican-3, and lack of reactivity with PAX-8 and EMA.
Figure 1A: Portion of the tumor demonstrating a classical morphology of clear cell carcinoma with round and irregular glands lined by a single layer of tumor cell showing clear cytoplasm, separated by a hyalinized stroma (Hematoxylin and Eosin, original magnification × 100).
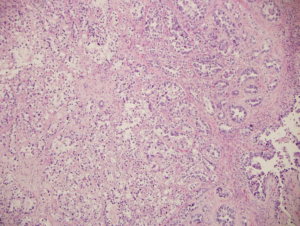
Figure 1B: Higher magnification showing hobnail cells with high nuclear grade, commonly seen in clear cell carcinoma. Hematoxylin and Eosin (original magnification × 400).
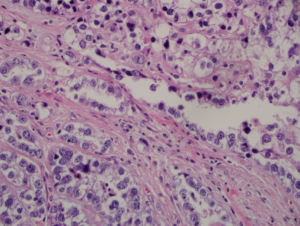
Figure 2A: Yolk sac tumor with non-specific glandular differentiation and Schiller-Duval bodies in a reticular background (left off-center and top mid-portion) adjacent to tubules, small glands and cysts of clear cell carcinoma (original magnification × 100).
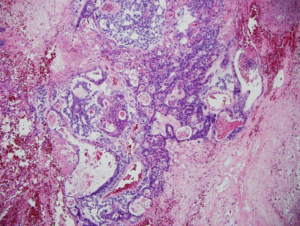
Figure 2B: Immunohistochemistry showing embryonal carcinoma with SALL-4 positive, HNF-1β negative tumor cells (original magnification × 100).
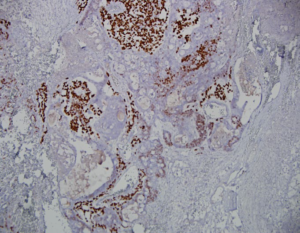
Figure 2C: In contradistinction to the adjacent clear cell carcinoma areas with SALL-4 negative, HNF-1β positive tumor cells on the right (original magnification × 100).
DISCUSSION
Review of the literature identified no other cases of ovarian cancer that matched the histology of the patient’s tumor. A case of a malignant ovarian tumor composed of endometrioid adenocarcinoma, clear cell adenocarcinoma, squamous cell carcinoma, yolk sac tumor and immature teratoma1 was, however, found in our PubMed search for “ovarian” and “YST” and “CCC.”
When assessing potential chemotherapeutic regimens, the standard therapies for both tumor histologies were reviewed. The chosen treatment plan was crafted based on the aggressiveness of the tumors and the intensity of the standard combination chemotherapies involved. YST is the second most frequently diagnosed ovarian germ cell tumor, and presents in young women and rarely in postmenopausal patients. Yolk sac tumor histology in postmenopausal women might increase the neoplasm’s resistance to chemotherapy and therefore predict a poorer prognosis.2 The patient began treatment with three cycles of BEP and one cycle of Etoposide and Cisplatin to aggressively address the YST component of the tumor. After the third cycle of chemotherapy, the patient presented with blisters on her hands and feet, fatigue, and depression. It was determined that the patient’s dermatologic symptoms were a result of Bleomycin. The most pragmatic treatment of intermediate to poor-prognosis metastatic germ cell tumors involves optimal cytoreductive surgery followed by four cycles of BEP.3,4 The patient was encouraged to substitute a single cycle of Etoposide and Cisplatin instead of foregoing the final cycle of BEP.
The second tumor histology, CCC, is commonly treated with six cycles of Carboplatin/Paclitaxel combination therapy. She was only able to complete 2 out of the planned 4 cycles because of the overlapping of families of chemotherapeutic agents between the two utilized regimens. Patient prognosis after receiving Carboplatin/Paclitaxel is similar in patients with CCC and patients with other epithelial ovarian histologies, but were poor when treatment was administered in the advanced stages of disease.5
In summary, this case is unique in that both YST and CCC tumor histology were identified in the left ovary of the patient. None of the aforementioned cases detail cases with this particular combination of tumor representation. Continued treatment of this patient’s ovarian neoplasm will hopefully provide more insight as to how to treat similar tumors in the future.
MAJOR CONCLUSIONS
The findings of co-existing germ cell and epithelial histology are unique in malignant germ cell tumors of the ovary and may require combination chemotherapy targeted towards both components.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
The patient has provided written permission for publication of the case details.










