INTRODUCTION
Cisplatin is a highly potent cytotoxic and genotoxic agent used in the chemotherapy for various types of cancers such as gastric cancer, which is one of the most malignant cancers in China.1,2,3,4,5 Apoptosis is an important pathogenic mechanismin solidary tumor,6 which is characterized by morphological changes such as cell shrinkage, chromatin condensation and fragmentation of DNA. Cisplatin treatment in vivo and in vitro results in apoptosis of cancer cells, which usually in dose- and time-dependent manner.
Several mechanisms are believed to mediate cisplatin-induced apoptosis. The primary mechanism of the cytotoxic effects is due to coordinative bonding between the atoms of platinum and DNA, leading to formation of intra-strand DNA cross-links, which induces apoptosis by activating p53 and cell cycle arrest.7 Previous study has been shown that Reactive Oxygen Species (ROS) generated by cisplatin increases lipid peroxidation, which alters enzymes and structural proteins, and direct the cell to an apoptotic pathway.8 Also, cisplatin-induced apoptosis could involve in the inflammatory pathway.9,10,11 Moreover, previous studies revealed that cisplatin induces apoptosis through a mechanism relaying on the Bax translocation into mitochondria and Caspase activation.12
Mitogen-activated protein kinases (MAPKs) are serine/threonine kinases involved in the regulation of various cellular responses, such as cell proliferation, differentiation and apoptosis. Three major mammalian MAPK subfamilies have been described: the Extracellular signal-regulated kinases (ERK), the c-Jun N-terminal Kinases (JNK), and the p38 kinases. Each MAPK is activated through a specific phosphorylation cascade. The ERK pathway plays a major role in regulating cell growth and differentiation, being highly induced in response to growth factors and cytokines. It is also activated by some conditions of stress, particularly oxidant injury, and such circumstances is believed to confer a survival advantage to cells.13 Comparatively, JNK and p38 are weakly activated by growth factors, but are highly activated in response to stress signals including tumor necrosis factor, ionizing and short wave length Ultraviolet irradiation (UVC), and hyperosmotic stress. Their activation is most frequently associated with induction of apoptosis.14,15,16 In cisplatin treatment, ERK was activated in a variety of cell lines. While some studies showed that the ERK activation enhances survival of cisplatin-treated cells,13 then some other studies reported that it induces apoptosis in cisplatin exposed cells.17,18,19,20,21,22 Gastric cancer is one of the malignant cancers in China. The functional roles of ERK in cisplatin treatment of gastric cancer remain elusive. The present study sought to delineate the role of the ERK signaling in cisplatin-induced apoptosis using a gastric cancer cell line, MKN-28.
MATERIAL AND METHODS
Cell Culture
Human gastric adenocarcinoma (MKN-28) cells were obtained from the American Type Culture Collection. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Hyclone, Logan, Utah, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah, USA) and 1% penicillin–streptomycin (Gibco, Grand Island, NY, USA). Cells were cultured in a 37 °C humidified incubator with a mixture of 95% air and 5% CO2.
Reagents
Constitutively Active-MEK1 and Domain NegativeMEK1 was purchased from Addgene, and then sub-cloned into pCMV vector. The MEK inhibitor U0126 and JNK inhibitor SP600125 were purchased from Tocrics (Bristol, UK). Immunoblotting experiments were performed with rabbit anti-caspase-3, anti-caspase-8, anti-caspase-9, anti-PARP, anti-Bid, anti-BAK, anti-Bax, anti-PUMA, anti-cytochrome c and anti-BCL-2 were from Epitomics (Burlingame, CA, USA), anti-phospho-p44/42 MAPK (p-ERK1/2) (Thr202/Tyr204), and anti-ERK1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA); The Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG polyclonal antibodies were obtained from Santa Cruz Biotechnology (Dallas, Texas, USA).
Immunoblotting (Western Blot Assay)
Cells were lysed in PIRA buffer (Beyongtime, China). Following boiling for 5 min, samples were separated on 10% SDS-polyacrylamide gels and then transferred to an Immobilon membrane (Millipore, MA, USA). The membranes were blocked with 5% non-fat dry milk in TBS/Tween20 (0.05% v/v) for 1 h followed by incubation at 4 °C overnight with the indicated primary antibodies. The membranes were washed three times with TBST buffer and then incubated for 1 h with HRP-conjugated anti-rabbit or -mouse secondary antibodies. Visualization of protein bands was accomplished using enhanced chemiluminescence (Thermo Scientific).
Detection of Cell Apoptosis (Annexin V/PI)
Cells were seeded at a density of 1.0×105 cells per well in 24-well plates, cultured with different reagents for 48 h, and then harvested. The cells were washed with PBS and then stained 5 μL of Propidium Iodide (PI) for 15 min followed by 5 μL of Annexin V and at room temperature in the dark according to the manufacturer’s instructions (Nanjing Key Gen Biotech, Nanjing, China). The apoptosis rate (%) of the stained cells was analyzed using FCM. The experiments were repeated three times.
Detection of Cell Death (Lactate Dehydrogenase Release
Cell death was quantitively detected based on the release of LDH by using a cytotoxicity detection kit (Roche Life Science, IN, USA) as described in manufacturer’s instruction. All the experiments were repeated three times and the average is shown in each figure.
Analysis of Cytochrome c Release
To assess mitochondrial cytochrome c release, cytosolic protein extracts were obtained according to instructions of a commercial cell fractionation kit (Enzo Life Sciences, Farmingdale, NY, USA). Briefly, 1 × 107 cells in a dish of 10 cm in diameter were washed twice with cold PBS and then centrifuged at 600g for 5 min at 4 °C. Cells were suspended in 0.1 ml of icecold fractionation buffer and then incubated on ice for 10 min before being homogenized with a syringe needle. The homogenates were centrifuged at 700 g for 10 min at 4 °C, after which the supernatants were collected and centrifuged at 10,000 g for 30 min at 4 °C. The supernatant was again collected as the cytosolic fraction and analyzed for cytochrome c release by western blot assay.
Silence BCL-2 with Smart Pool siRNA
SMART pool: ON-TARGET plus BCL-2 siRNA and Transfection Reagent-1 were both purchased from Damarchon (Lafayette, CO, USA), Cat# L-003307-00-0005. Transfection procedure was followed the manufacturer’s instructions.
Statistical Analysis
All results of bar graphs are expressed as the mean ± S.D. obtained from three independent experiments in duplicate. Statistical differences were evaluated using the Student’s t-test or ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Cisplatin Induces Apoptosis in MKN-28 Cells through the Mitochondrial Pathway
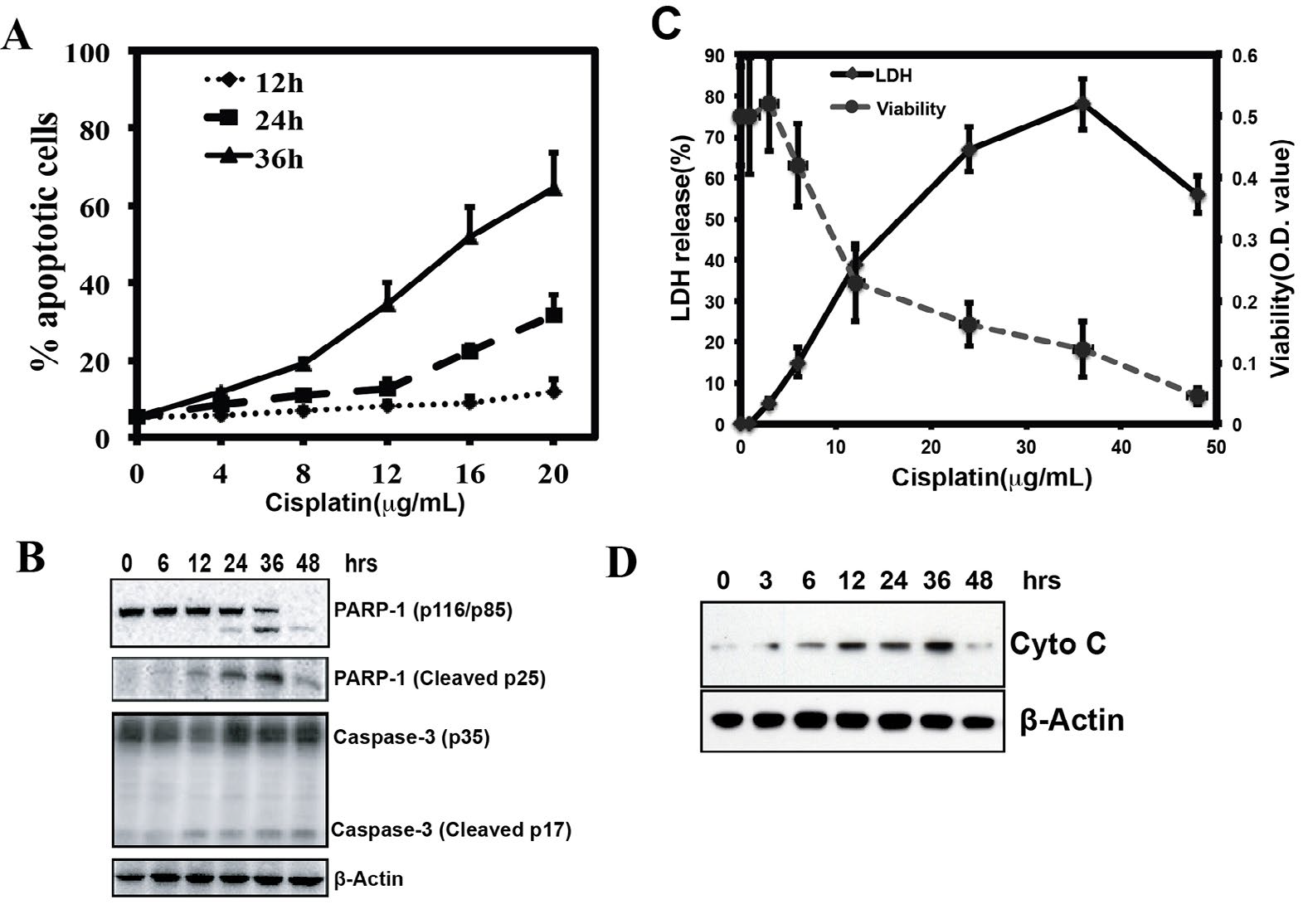
Cisplatin treatment induces apoptosis in many different cell lines. To test the effect of apoptosis in MKN-28 cells, MKN-28 cells were treated with cisplatin in an increasing dose (0 μg/mL, 4 μg/mL, 8 μg/mL, 12 μg/mL, 16 μg/mL, and 20 μg/ mL) for different time point (12 h, 24 h, 36 h). After the treatment, cells were stained by Annexin V-FITC and PI, and were examined by Flow cytometry (FCM). As shown in figure 1A, cisplatin treatment resulted in dose-and time-dependent manner in MKN-28 cells. Most cells were dead after 48 h treatment in dose 16 μg/mL and 20 μg/ml (Supplementary Figure 1). The apoptotic marker such as cleaved caspase-3 and cleavage of Poly (ADP-ribosyl) polymerase (PARP) can be detected by Western blot analysis (Figure 1B). The LDH releasing experiment also revealed that the LDH leaked from apoptotic cells after cisplatin treatment (Figure 1C). Meanwhile, the cell viability was opposite changed. Treatment of MKN-28 cells with cisplatin resulted in a time-dependent increase in release of cytochrome c from mitochondria (Figure 1D). These results suggested that cisplatin induced MNK-28 cell apoptosis via the mitochondrial pathway.
Supplementary Figure 1: Annexin V/PI apoptosis assay used to detected apoptosis of MKN28 cells treated with Cisplatin in different doses for 48 hours. Most cells died at 16 and 20 μg/mL concentration.
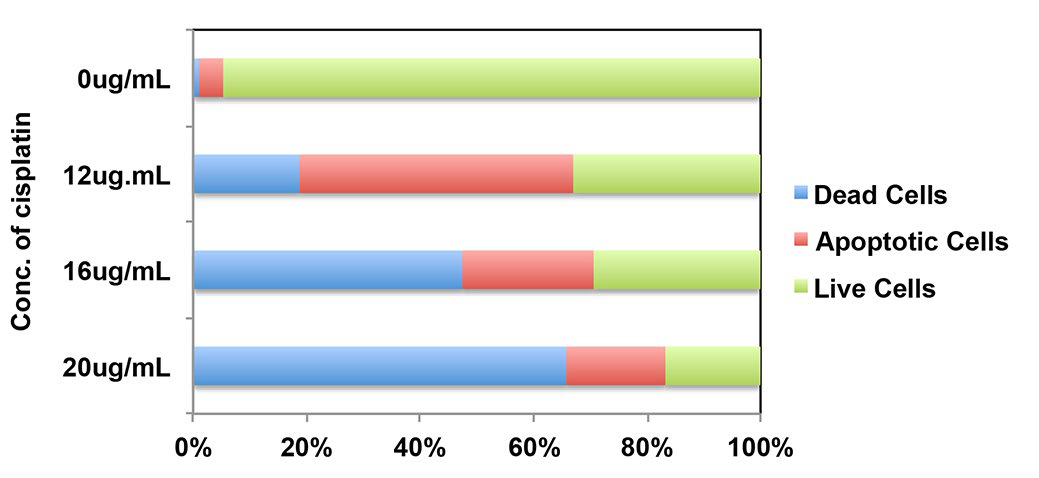
Figure 1: Cisplatin induces apoptosis of MKN-28 cells via intrinsic pathway. (A) Annexin V/PI apoptosis assay used to detected apoptosis of MKN-28 cells treated with Cisplatin in different doses and time. It showed a time and dose dependent manner (*p<0.05, **p<0.01). (B) Immunoblot analysis of the indicated caspase substrates within lysates generated from MKN-28 cells that were untreated or were cultured for 6 h, 12 h, 24 h, 36 h and 48 h in the presence of Cisplatin (20ug/ml). (C) Cytotoxicity was calculated by measuring the amount of LDH released from the cytosol of asbestos-damaged cells into the supernatant. MTT assay: The OD value of 540 nm, which reflects metabolically active cells, decreased proportionally when cells were exposed to increasing amounts of cisplatin. (D) Mitochondrial release of cytochrome c release after treated with 20 ug/ml cisplatin at 0h, 3 h, 6 h, 12 h, 24 h, 36 h and 48 h.
Cisplatin Induces Apoptosis in MKN-28 Cells with the MAPK
Signaling Pathway activation
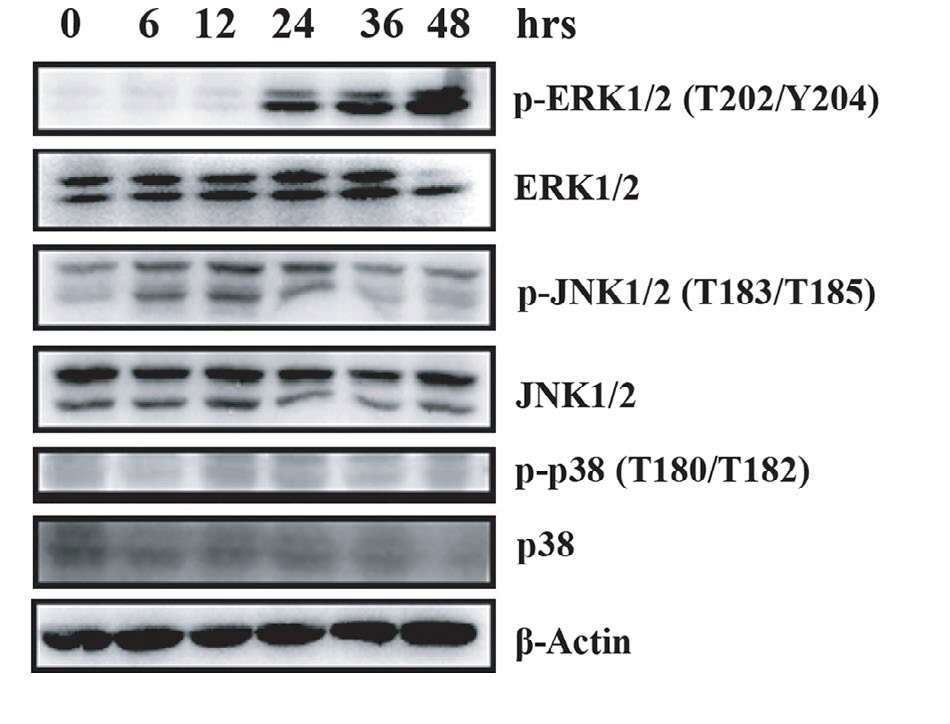
Cells were exposed in cisplatin at various time points in a dose of 20 μg/ml. The activation of MAPK pathway was then detected by Western blot, including ERK1/2 (p42/44), p38, and JNK. In the treatment, JNK was activated moderately while ERK was activated remarkably. (Figure 2) This result showed a relationship between cisplatin-mediated apoptosis and ERK signaling cascade.
ERK activation enhanced cisplatin-induced apoptosis
Pharmaceutical inhibition was applied to test if the MAPK/ERK pathway involved in cisplatin-induced apoptosis. The effect of MAPK inhibitors was detected with Western blot (Figure 3A). Apoptosis assay analysis showed that cisplatininduced apoptosis of MKN-28 cells was blocked by the MEK inhibitor U0126, but not c-Jun N-terminal Kinase (JNK) inhibitor SP600125 at the 24 hr and 36 hr. (Figure 3B). These results demonstrated that the cisplatin-induced apoptosis with MAPK pathway activation. It suggested that cisplatin-induced MKN-28 apoptosis was abolished by the MEK inhibitor U0126, but not by JNK inhibitor SP600125.
In addition, we test the role of ERK by ectopically expressing constitutively active and dominant-negative ERK mutant. Cells were transfected with pCMV-CA-MEK1 and pCMVDN- MEK1 (Figure 3C). Highly activation of phospho-ERK was detected by Western blot after transiently transfected CAMEK1, but not for the DN-MEK1 transfected cells. When these cells were treated with cisplatin, CA-MEK1 transfected cells presented higher activation than control group while a reduced level of ERK activation appeared in dominant-negative mutant expressing cells (Figure 3D). The apoptosis assay suggested that CA-MEK1 group acquired higher ERK1 activation and apoptosis (Figure 3E and 3F). These data indicate that ERK activation induces the cisplatin-induced MKN-28 cells apoptosis.
Figure 3: Cisplatin-induced apoptosis was prohibited by MEK inhibitor, whereas enhanced by CA-MEK1 transfection. (A) Representative Immunoblot of MAPK activation of untreated MKN-28 cells, MKN-28 cells treated with 20 ug/ml Cisplatin, MKN-28 cells treated with 10 μM U0126 plus 20 ug/ml cisplatin, and MKN-28 cells treated with 20 μM SP600125 plus 20 ug/ml cisplatin and two inhibitors combination with 20 ug/ml cisplatin for 36 h. Phospho-ERK1/2, Phospho-JNK1/2 and total protein were examined. (B) Quantitation of apoptosis used Annexin V/PI FCM assay. (C) Immunoblot assay was used to detect the ERK activation of MKN-28 cells after CA-MEK1 or DN-MEK1 transient transfection. (D) The control and transfected MKN-28 cells treated with 20 ug/ml cisplatin for 36 h, the phosphor-ERK1/2 was detected by immunoblot assay. (E, F) The apoptosis of the control and transfected MKN-28 cells, which incubated with or without cisplatin, were calculated by Annexin V/PI FCM apoptosis assay (*p<0.05, **p<0.01). Each experiment was repeated for at least three times.
ERK-Mediated BCL-2 Inhibition Induces Apoptosis
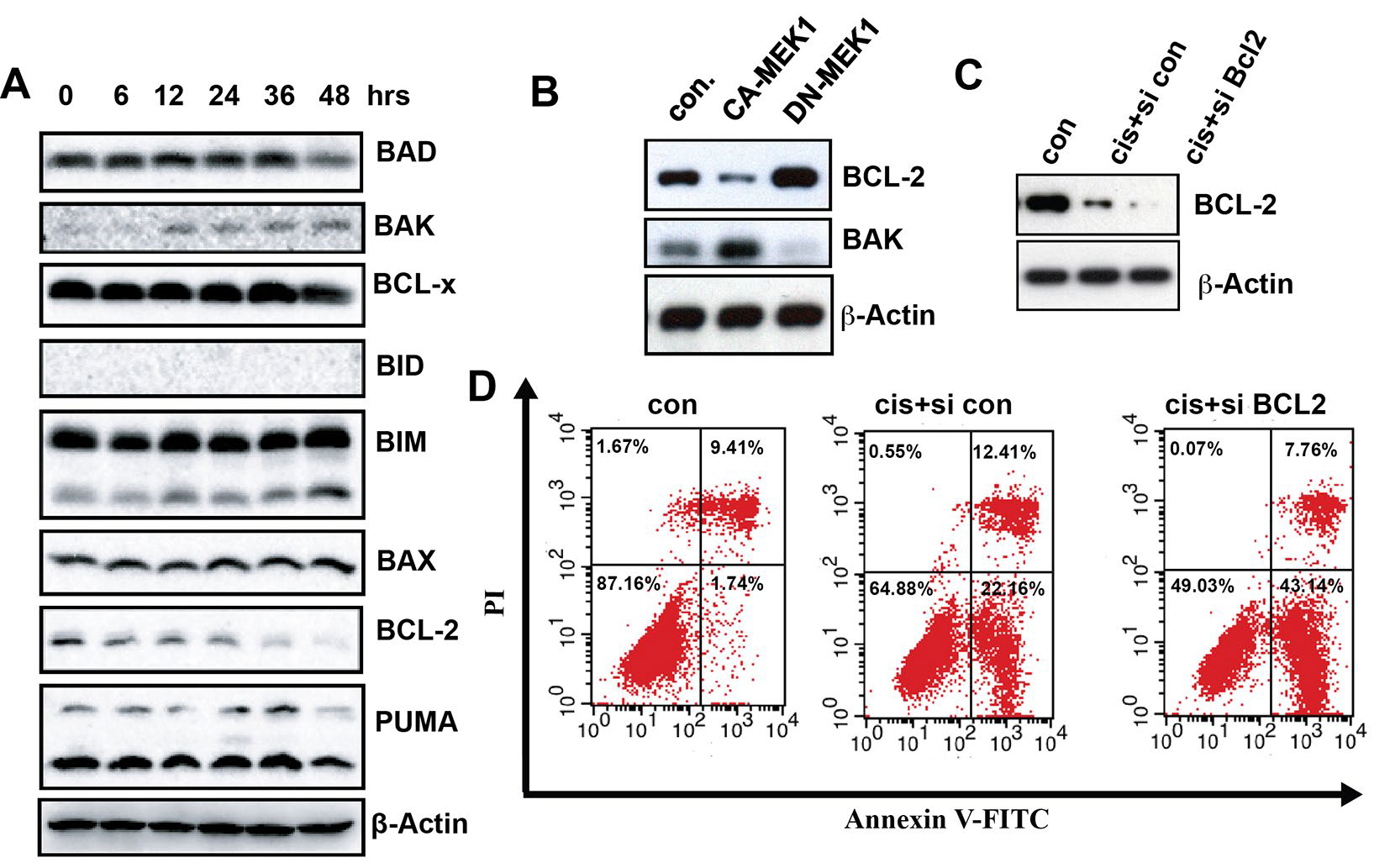
To examine the relationship between ERK activation and BCL-2 family, we investigated the BCL-2 family by Western Blot. After cisplatin treatment, the BCL-2 expression level was down-regulated. Meanwhile, the BAK level was up-regulated (Figure 4A). To test if BCL-2 is the downstream transducers of the activated ERK-induced apoptosis, MKN-28 cells were transient transfected with CA-MEK. Dramatically, we found that BCL-2 was down-regulated concurrently with BAK upregulation after CA-MEK1 transfection. The opposite result can be found after DN-MEK1 transfection (Figure 4B). This result suggested that BCL-2 and BAK are both downstream of activated ERK. Furthermore, when BCL-2 was knocked down by smart pool siRNA, cisplatin-induced apoptosis was remarkably increased (Figure 4C and 4D). Taken together, the results suggested that, with cisplatin treatment, (1) ERK activation regulate BCL-2 and BAK, (2) BCL-2 down-regulation facilitate apoptosis.
Figure 4: BCL-2 and BAK are the downstream transducers of ERK, which enhanced cisplatin-induced MKN-28 cells apopotosis. (A) Immunoblot analysis of the BCL-2 family within lysates generated from MKN-28 cells that were untreated or were cultured for 6 h, 12 h, 24 h, 36 h and 48 h in the presence of cisplatin (20 ug/ml). (B) Immunoblot assay was used to detect the BCL-2 and BAK protein expression level in MKN-28 cells that transient transfected CA-MEK1 or DN-MEK1. (C) Immunoblot assay was used to detect the BCL-2 and BAK protein expression level in MKN-28 cells that transfected with smart pool siRNA for BCL-2 after 20 ug/ml cisplatin treatment. (D) The apoptosis of the control and MKN-28 siBCL-2 cells, which incubated with or without cisplatin, were calculated by Annexin V/PI FCM apoptosi.
DISCUSSION
Two major distinct apoptosis pathways have been described for mammalian cells: (1) Mitochondrial independent pathway, which is recruited by the adapter molecule Fas/APO-1- -associated death domain protein to death receptors upon extracellular ligand binding;23,24 (2) cytochrome c release-dependent activation of caspase-9 through Apaf-1.25,26,27 We did, however, observe an increase of cytochrome c in the cytoplasm of cisplatin-treated cells relative to untreated cells, suggesting that cytochrome c release is involved in cisplatin-induced apoptosis.
The MAPK pathways, including ERK, were typically studied previously. However, the roles of ERK involved in cancer protection are controversial.28,29,30Most investigations revealed the importance of MAPK signaling pathways in regulating apoptosis during conditions of stress. But majority of the studies supported the general hypothesis that ERK activation delivers a survival signal and inhibition of ERK signaling leads to increased sensitivity of some cell lines to cisplatin.16,31,32Although more often associated with survival, a pro-apoptotic function for the ERK pathway has also been suggested in several other model systems.21,22 In our study, however, we provided evidence that activation of ERK is important for the inducting cisplatin-induced apoptosis in MKN-28 cells. By modulating ERK activity, we found that both Domain-Negative MEK transfection and MEK specific inhibitor U0126 led to an inhibition of cisplatin-induced apoptosis. Meanwhile, enhancement of ERK activity by overexpressing Constitutively Active MEK in MKN-28 cells accentuated cell apoptosis. ERK activity-dependent cisplatin-induced apoptosis was not limited to MKN-28 cells, but also occurred in human lung cancer A549 cells and Hela cells.17,21
Although several studies have shown that JNK is activated in cisplatin treatment, their role in determining survival is unclear. A number of studies have provided evidence indicating that the JNK pathway contributes to cisplatin-induced apoptosis.15,16 However, others have suggested that JNK signaling is important for cell survival.11,33,34 Such differential effects observed from one study to another could indicate cell type specificity. In our own studies using MKN-28 cells, we found that JNK was indeed activated by cisplatin treatment. However, down-regulation of this pathway by JNK specific inhibitor SP600125 did not made any changes of the sensitivity to cisplatin. Thus, only ERK appears to play a major role in influencing the survival of cisplatin-treated MKN-28 cells.
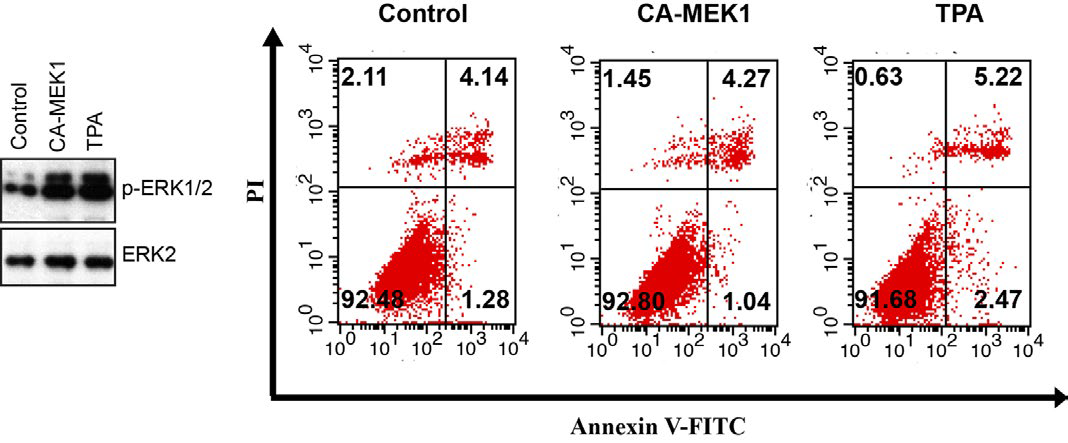
How does ERK activation lead to apoptosis of cisplatin-treated cells? What are the downstream transducers involved in mediating the apoptosis? Based on previous and our study, ERK activation is necessary, but not sufficient to induce apoptosis, as TPA treatment (Supplementary Figure 2) or constitutively active-MEK over expression did not result in cell death. With cisplatin, no significant activation occurs until 6 h, but the activity remains highly elevated through the remaining time period examined (48 h).
Supplementary Figure 2: Immunoblot assay was used to detect the ERK phosphorylation in MKN-28 cells that transfected with CA-MEK1or treated with TPA without cisplatin treatment. The apoptosis of MKN-28 control cells, CA-MEK1transfected cells and TPA treated cells were calculated by Annexin V/PI FCM apoptosis assay. Each experiment was repeated for at least three times.
BCL-2 is known as an anti-apoptotic BCL-2 family member, for which amplification has been associated with a more aggressive malignant phenotype and drug resistance to various categories of chemotherapeutic drugs in malignancies.35 Moreover, BCL-2 was reported as a target gene for many signaling cascades, such as Phosphatidylinositol 3 kinase (PI3K)/ nuclear factor-κB (NF-κB) signaling pathway, the cyclic AMP (cAMP)/protein kinase A (PKA) cascade, MAPK cascade and so on.36,37 In this study, we found that treatment of cisplatin increased phosphorylation of ERK proteins, which intervened downstream anti-apoptosis molecular BCL-2 and up-regulation of pro-apoptosis molecular BAK in gastric cancer MKN-28 cells. With BCL-2 and BAK change, cisplatin promoted MKN28 cells apoptosis.
Increasing amount of evidence indicated that the ERK pathway is important in malignant disease.38,39 One recent study indicated that ERK activation by TGF-beta plays a key oncogenic role in Colorectal cancer (CRC) with SMAD4 inactivation mutations. The MEK inhibitor U0126 can surprisingly reduce CT26 cells tumor genesis and liver metastasis in vivo and in vitro, 40 suggesting that MEK inhibitors can be a primary approach for treatment of human malignancies. However, our findings indicated a need for caution for the generality of this therapeutic approach. On the other hand, coupled with previous studies,21,22 our findings supported a role of the ERK signaling pathway in enhancing cisplatin-induced apoptosis, further suggesting that strategies targeting activation of this cascade could be employed to enhance the therapeutic effectiveness of cisplatin. In addition, evaluation of ERK activity could be useful marker for predicting which tumors would present favorable response to cisplatin therapy.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We should thank all the staffs in my lab for discussing the project. This work was supported by the State Key Project on Infection Disease of China (Nos. 2012ZX10002016-004 and 2012ZX10002010-001-004). The National Natural Science Foundation of China (81202300 and 81372327).










