BACKGROUND
Dietary therapy through a low-protein diet (LPD) has long been used to preserve the renal function of patients with chronic kidney disease (CKD). 1,2,3 LPD restricts the amount of protein in the total dietary intake and it lowers the production of urea nitrogen, an end product of protein metabolism.4 It is considered that the level of protein intake modulates the filtration load on the kidneys and tempers the decline of renal function.5,6 Over the past 50 years, LPD have been successfully used to treat chronic renal failure in Japan.7 Since the easiest course of action is to cut down on foods which are high in protein, the main dishes, such as meat and fish, are usually reduced to smaller servings. However, such frugality compromises the quality of meal times. Patients may have difficulty adhering to the LPD, and as a result, be unable to reap the benefits of LPD.
Removing protein from rice would minimize the need to cut down on main dishes.
Mealtime quality would be maintained, making it easier for patients to follow the LPD.
To address this issue, in 1992 the Niigata Agricultural Research Institute Food Research Center developed a technology (JP2706888B) using lactobacillus fermentation to reduce the protein, phosphate, and potassium contained in white rice (WR).8 However, their method posed technical challenges such as extremely long processing times and the difficulty of microorganism control hampering reliable production. In 1994, Forica Foods Co., Ltd., Uonuma City, Niigata, Japan built on this technology to develop an exclusive proteolysis technology (JP3156902B) utilizing an enzyme product which can break down protein in WR more quickly and reliably than lactobacilli fermentation.9 The following year, the manufacture and sales of “Cooked PLC Rice 1/3 (PLC: Protein Low Content)” began.
PLC Rice is processed, protein reduced WR. The palatability (quality) of this low-protein rice (LPR) is such that it can be eaten day after day. The PLC Rice products were developed to facilitate adhesion to the LPD, thereby improving the quality of life (QoL) of patients with CKD. In this article, the manufacturing process for the PLC Rice series will be described.
METHODS
Raw Materials and Protein Digestion
Raw materials are WR, pH adjuster, and protease.
There is no particular restriction on the type of WR. For example, either Japonica rice (short grain) or Indica rice (long grain) can be used. Highly clean raw material is used to reduce the risk of microbiological contamination.
In the protein digesting step, the temperature and pH must be maintained for a certain amount of time at levels conducive to enzyme activity. To prevent in-process microbiological proliferation and consequent product contamination, it is desirable to keep the reaction solution pH acidic and at or above 50 °C. To control pH, a suitable quantity of organic acid is added. Suitable organic acids include citric acid, lactic acid, fumaric acid, gluconic acid, and glucono delta-lactone (GDL). Out of these, citric acid is chosen because of its high buffering property, ability to maintain pH within the enzyme solution stably over a long period, and for having the smallest negative effect on the taste and aroma of cooked rice.
As proteolytic enzyme, acidic protease approved by the Ministry of Health, Labour and Welfare (MHLW) as a food additive and available on the market is used. In our method, we use an enzyme mix (EM) consisting of an exclusive formulation of at least 3 kinds of enzyme products, comprising at least 1 each derived from Aspergillus oryzae, Rhizopus niveus, and Aspergillus niger respectively. Each enzyme product is a crude enzyme product extracted from the source microorganisms, and the main component is protease in the aspartic protease family (EC 3.4.23).
Protein Digesting Process
The protein digesting process is broadly divided into enzyme digestion and washing. In the first step, surface bran residue and germs were washed away with water. The washed WR is placed in a reaction solution containing dissolved EM and citric acid, and allowed to soak for a certain period of time (up to 24 hours) in a temperature and pH conducive to enzyme activity. This breaks down the protein in the WR. Subsequently, thorough washing removes any unnecessary matter: rice protein decomposition product, EM, and excess citric acid. These steps yield LPR (Raw LPR), which is raw, protein reduced WR.
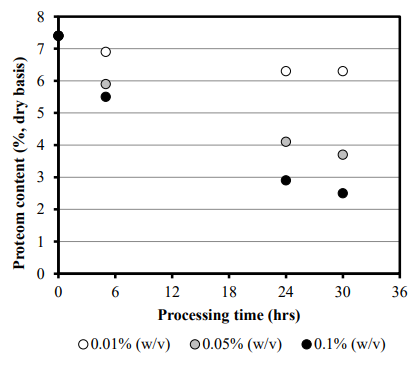
Figure 1 shows the time lapsed changes of protein mass in rice treated with enzyme with a single protease derived from Aspergillus oryzae. Protein mass in rice is highly dependent on enzyme concentration and reaction time. By adjusting these factors, the rice protein mass can easily be reduced to target levels. In the actual process of protein enzyme treatment, EM is used instead of a single enzyme, resulting in better removal rates and production efficiency compared to a single enzyme. This is likely to be because the combination of multiple enzymes each with different protein cleavage sites reduces the molecular weight of the decomposition product. The smaller the molecular weight of rice protein, the easier it is to elute from the rice grain.
Figure 1. The Effect of Processing Time and Enzyme Concentration on Protein Removal.
Manufacturing Method
The manufacturing flow of these products is outlined in Figure 2. Regardless of product form, low protein rice products need to have their rice protein content broken down and removed. The method employed by Forica Foods is based on the invention by Nakajo et al (JP3156902B), and consists of utilizing protease to process protein.9 The very simple production process is shared across product forms.
Figure 2. Production Flow Chart of PLC Rice.
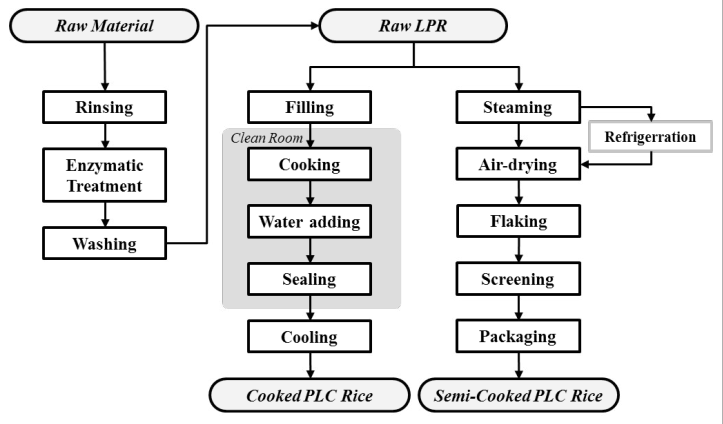
Cooking Process
The Raw LPR obtained by said protein digestion process requires special techniques and know-how to produce rice grains which maintain their shape while offering palatability. This is because Raw LPR grains are extremely fragile, and when subjected to steaming or cooking like ordinary WR, they dissolve into a gruel-like mass which is far from delicious. In order to solve this problem, we developed a method (WO 2017037799 A1) for processing LPR into a palatable, high quality product.10
Characteristics of the Manufacturing Method for “Cooked PLC Rice”
The manufacturing method for “Cooked PLC Rice” can be broadly categorized into cooking, hot water injection, sealing, and cooling. In the first step, each serving of the Raw LPR is filled into heat resistant containers. Without adding water, the containers are transported to our exclusive rice steamers installed in clean rooms. The rice steamer chamber is sealed and the interior de-aired to create a vacuum. Immediately, steam is injected powerfully to instantly raise the temperature within the chamber to 130 °C to 140 °C. This state maintained for 30 to 120 seconds during which the rice is steamed rapidly. Immediately after steaming the rice, sterile hot water of at least 90 °C is added. The steamed LPR is promptly sealed by heat resistant film with oxygen absorber affixed to it. The temperature is maintained for an additional 5 to 10 minutes to ensure thorough steaming of the content. Subsequently, the packaged rice is allowed to air cool gradually for 20 to 30 minutes until its temperature reaches 30 to 35 °C, then subjected to X-ray inspection to check for foreign matter and weighed. Articles which pass the inspections become product.
Using the steaming method described above, in which rice is steamed by elevating the temperature in the chamber instantaneously without adding water, the starch can be prevented from eluting from the Raw LPR, thereby avoiding loss of the flavor of white rice and making it possible to obtain low protein rice with appropriate viscosity and elasticity that is suited to Japanese preferences. In addition, the hot water injection process makes it possible to change the amount of added water at will. Thus, the moisture content of the product can be adjusted easily to obtain the desired hardness/softness of the cooked rice. As has been described, this technique is an extremely unique method of cooking rice which offers both practicality and flexibility.
Characteristics of the Manufacturing Method for “Semi-Cooked PLC Rice”
The manufacturing method for “Semi-Cooked PLC Rice” can be broadly categorized into steaming, drying, and flaking to separate into individual grains. The most basic manufacturing method consists of spreading out Raw LPR into a uniform thickness, steaming with steam, then loosely separating the grains, drying uniformly overall, then flaking to separate the rice grains into individual grains. By gelatinizing and then drying the raw starch contained in Raw LPR, it is possible to obtain “Semi-Cooked LPR” whose rice grains are strong enough for long-term storage and which can be cooked similarly to WR.
However, simply steaming the rice with saturated steam results in highly viscous starch eluting from the surface of the LPR grains. This causes the LPR grains to stick strongly to each other, making it extremely difficult to separate the grains into uniform size. Variations in the size of LPR clusters can cause uneven drying. Trying to separate unevenly dried LPR clusters generates large quantities of cracked and crushed rice, resulting in significant loss of productivity. Furthermore, when LPR thus obtained is cooked, it is stickier than necessary, compromising the quality of the product.
To solve the issues of productivity and reliable product quality, we invented the 2 processing methods shown below.
Refrigeration to retrograde the starch: Raw LPR is spread out evenly into a thickness of 30 to 50 mm, then steamed for 5 to 10 minutes with saturated steam. This is transferred to a refrigerator and kept at 0 to 5 °C for 24 to 72 hours. This causes the starch, gelatinized by steaming, to retrograde, resulting in weakened adhesion between rice grains, thereby facilitating the flaking process. After the grains are separated, the rice is uniformly dried to bring the moisture content of the grains to 15 to 26%. The separated LPR grains are screened for grain shape and color, and the grains with uniform shape and color are weighed and sealed in packaging material together with oxygen absorber to obtain product.
Superheated steam: Raw LPR is spread out evenly into a thickness of 30 to 50 mm, then steamed for 4 to 10 minutes using superheated steam of at least 105 °C. The superheated steam, obtained by heating saturated steam, makes it possible to heat efficiently using higher temperatures than saturated steam. Furthermore, superheated steam is drier than saturated steam, thereby keeping down excess moisture in the rice during steaming. Therefore, the steamed rice is less viscous and more easily separated.
The subsequent steps are similar to method described above. Immediately after steaming, the Steamed LPR is separated loosely and dried by warm air so that the moisture level is uniform throughout, at 16% to 20%. The rice is then separated into individual grains using a cracking device, etc. The separated LPR grains are screened for grain shape and color, and the grains with uniform shape and color are weighed and sealed in packaging material together with oxygen absorber to obtain product.
Compared to method using retrogradation, in which the process is interrupted for the refrigeration step, this method consists of fewer steps and is amenable to continuous production conveyor-style. Therefore, it is possible to manufacture “SemiCooked PLC Rice” efficiently.
RESULTS
Nutrition
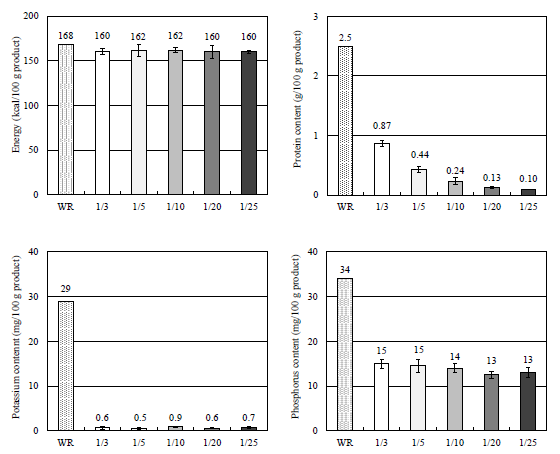
Nutrition information on PLC Rice products is shown in Table 1. Test samples were taken from at least 3 separate production days. As shown in the Table 2, there is minimal interlot variability of nutritional values, indicating extremely high reproducibility. Particularly noteworthy is the protein content, which demonstrates reliability in meeting the respective target values.
Table 1: Nutrient Content in 100 Grams of “Cooked PLC Rice” Products.
| Table 1: Nutrient Content in 100 Grams of “Cooked PLC Rice” Products. |
| Content |
Unit |
WR |
Mean (+SD)
|
| “1/3” |
“1/5” |
“1/10”
|
“1/20”
|
“1/25”
|
| Calories |
kcal
|
168 |
160 (3.1) |
162 (6.8) |
162 (2.6) |
160 (7.1) |
160 (1.7)
|
|
Moisture
|
%
|
60.0 |
60.4 (0.7) |
60.1 (1.7) |
60.1 (0.8) |
60.5 (1.6) |
60.6 (0.5) |
| Total Protein |
% |
2.5 |
0.87 (0.1) |
0.44 (0.0) |
0.24 (0.1) |
0.13 (0.0) |
0.10 (0.0)
|
|
Total Fat
|
%
|
0.3 |
0.5 (0.1) |
0.5 (0.1) |
0.6 (0.1) |
0.3 (0.1) |
0.5 (0.2) |
| Total Carbohydrate |
% |
37.1 |
38.1 (0.7) |
38.9 (1.7) |
39.0 (0.7) |
39.1 (1.4) |
38.8 (0.7)
|
|
Ash
|
%
|
0.1 |
0.1 (0.1) |
0.1 (0.1) |
0.1 (0.1) |
0 (0) |
0 (0.1) |
| Sodium |
mg |
1 |
2 (0.3) |
2 (0.0) |
2 (0.2) |
2 (0.1) |
2 (0.1)
|
|
Potassium
|
mg
|
29 |
0.6 (0.3) |
0.5 (0.2) |
0.9 (0.2) |
0.6 (0.1) |
0.7 (0.2) |
| Calcium |
mg |
3 |
5 (0.0) |
5 (0.3) |
5 (1.2) |
5 (0.7) |
5 (0.6)
|
|
Phosphorus
|
mg
|
34 |
15 (1.0) |
15 (1.5) |
14 (1.0) |
13 (0.7) |
13 (1.2)
|
Product Overview
PLC Rice is LPR which is processed, protein reduced WR (Figure 3). Some of the products in the series have been approved as “Food for special uses” by the Ministry of Health, Labour and Welfare, and for bearing claims on the label that they are suitable foods for kidney disease patients. In 1995, when we launched the first product, it contained 1/3 of the protein in WR, that is, 2/3 of the WR protein had been removed. With continued technological development, we achieved a reduction rate of 1/25 by 2007. As of 2017, 22 years from its development in 1994, the PLC Rice series consists of 10 products to accommodate consumer needs in terms of product form, reduction rate (1/3 to 1/25), and serving size (140 to 180 grams).
Table 2: The Effect of Processing Time and Enzyme Concentration on Protein Removal.
| Table 2: The Effect of Processing Time and Enzyme Concentration on Protein Removal. |
|
Protein content (%, dry basis)
|
| Processing time (hrs) |
Enzyme concentration (%, w/v)
|
|
0.01
|
0.05 |
0.1
|
|
0
|
7.4 |
7.4 |
7.4
|
|
5
|
6.9 |
5.9 |
5.5
|
|
24
|
6.3 |
4.1 |
2.9
|
Figure 3. The List of PLC Rice Products.
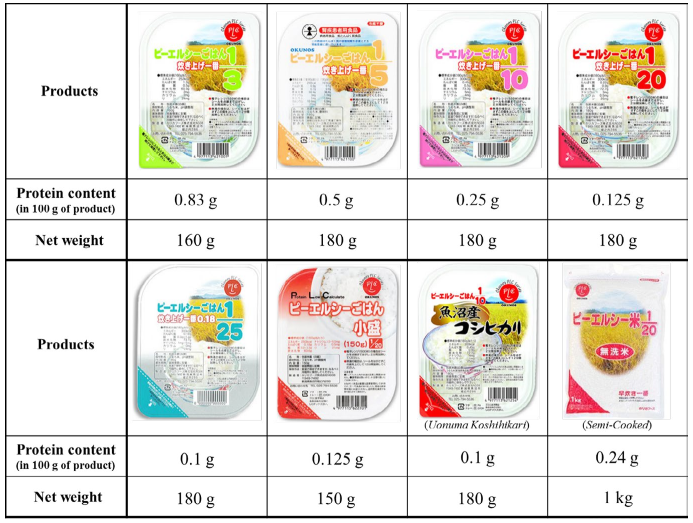
PLC Rice comes in 2 product forms: “Cooked PLC Rice” which can be microwaved or boiled in the pouch, and “Semi-Cooked PLC Rice” which requires cooking in a rice cooker. In “Cooked PLC Rice,” LPR is aseptically packaged. In “Semi-Cooked PLC Rice,” LPR is steamed, dried, flaked, and sieved, and requires a finishing step of cooking such as boiling, steaming, or microwaving before serving. Both types of products can be stored and distributed at room temperature. The best-before date of “Cooked PLC Rice” is 7 months and “SemiCooked PLC Rice” is 12 months.
Residual Enzyme in Product
Figure 4 shows the SDS-PAGE profile (CBB stain) of Raw LPR extract. Compared to extract from non-treated WR (L1), Raw LPR extract (L2) no longer shows any protein-derived bands except for some in the low molecular weight side. There were no bands common to Raw LPR (L2) and EM (L3), indicating that enzyme derived protein is absent (or under the CPB stain detection limit). These results show that rice protein decomposition product and EM are washed away from Raw LPR, leaving no residue in the product.
Figure 4: CBB-Stained SDS-PAGE Patterns of the Raw LPR Extract.
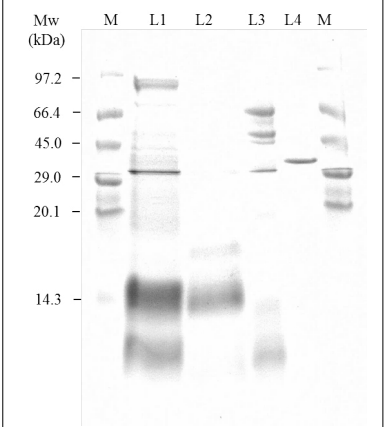
Microbiological Safety of Product
To store the packaged “Cooked PCR Rice” safely in room temperature, the contents must be sufficiently sterilized and sealed in the sterile state. Raw LPR is steamed with high temperature, high pressure steam in a steaming device installed in a clean room. At the same time, it undergoes thorough sterilization under conditions which are equivalent to the 120 °C for 4 minutes (F0 =3.1) stipulated in the Japanese Food Sanitation Act for retort pouch food. Subsequently, the contents are kept sterile while sealing together with oxygen absorber, thereby securing sterility of the product. In fact, since its adoption in 2004, there have been no incidents of product spoilage or deterioration attributable to insufficient sterilization, proving the efficacy and safety of this technique.
According to our findings relating to “Pre-Cooked PLC Rice,” drying steamed LPR to a moisture content of 15 to 26% yields desirable quality (particularly mouthfeel) when it is cooked. By sealing this together with oxygen absorber and maintaining an anaerobic state inside the package, it becomes possible to store it safely at room temperature. However, once the package is opened, new oxygen flows into the package. The anaerobic state cannot be maintained and there is risk of mold and other microbiological growth. A product with moisture level higher than 20% requires refrigeration or freezing. However, a product dried to a moisture level of 16% to 20% can be stored safely at room temperature. This is due to the reduced water activity of the product, which prevents mold etc. from proliferating after the package is opened.
Product Safety and Quantities Manufactured
Quantities of PLC Rice produced from launch to the present day are shown in Figure 5. In more than 20 years since its launch, nearly 100 million PLC Rice products have been provided to CKD patients through many hospitals throughout Japan. Although sales have declined since the peak of 7.5 million servings in 2000, the quantities have been growing gradually since 2004 when the above-mentioned high temperature steaming method was adopted. As of 2016, we are producing the equivalent of 4.7 million servings annually.
Figure 5: The Production Amount of PLC Rice.
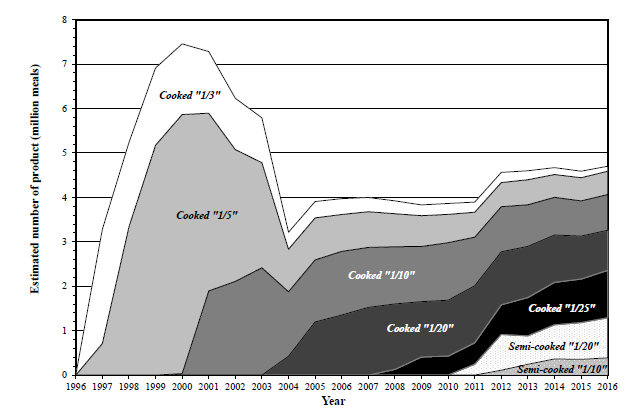
In the 22 years of producing and selling “Cooked PLC Rice” and the 7 years of “Semi-Cooked PLC Rice,” there have been zero reports of health problems attributable to these products. Given the high frequency of LPD Rice ingestion by CKD patients who eat it every day as part of their LPD, and the fact that PLC Rice products are composed almost entirely of natural ingredients derived from WR, it is reasonable to say that PLC Rice, regardless of product form, is safe to eat and has no negative health effects, even for CKD patients who ingest them repeatedly over the long-term.
DISCUSSION
From clinical findings, the recommended protein intake for Japanese CKD patients is under 0.5 g/kg body weight (BW)/day.11 The cooked rice that is the staple for Japanese people contains 2.5% protein. If a person eats a bowl (approximately 165 g) of cooked rice 3 times a day, he or she will ingest 12.5 g of protein from the rice. A patient weighing 60 kg should ideally ingest less than 30 g of protein per day. If the patient eats regular rice, it would account for 40% of the protein allowance. To keep with the allowance, the entire meal plan needs to be adjusted.
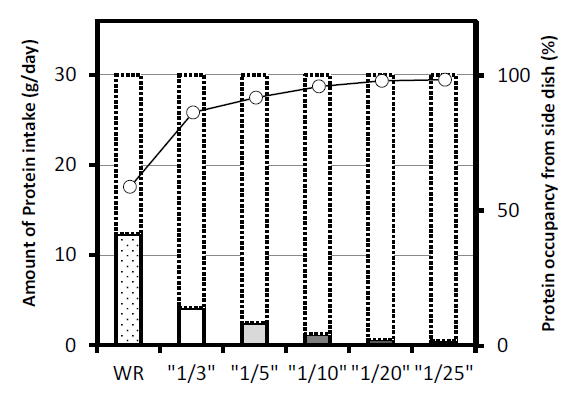
Figure 6 shows the how different proportions of a hypothetical patient’s daily protein allowance of 30 g is taken up by eating the same 165 g (1 serving) of WR or PLC as the staple food 3 times a day. Unprocessed WR translates to roughly 40% of the daily protein allowance, thereby leaving only about 60% to be allocated to side dishes (Figure 2). By contrast, LPR, even at the reduction rate of 1/3, only accounts for 8.4% of the daily allowance, leaving more than 90% to be occupied by other dishes. The smaller the reduction rate, the less protein from the staple, thereby permitting the rest of the allowance to be filled by other foods. This greatly expands the patient’s food choices.
Figure 6: Relationship between Total Protein Allowance and Protein Intake from Staple Out of Total Protein Allowance.
Bars with solid outline: Protein intake when ingesting 165 g (1 serving) of regular rice or LPR 3 times a day
Bars with dotted outline: Daily protein allowance (30 g/day) White circle and solid line: Difference and percentage – Total protein allowance and protein intake from staple out of total protein allowance
WR; White Rice, “1/3”; PLC Rice 1/3 (0.83 % protein), “1/5”; PLC Rice 1/5 (0.5 % protein), “1/10”; PLC Rice 1/10 (0.25 % protein), “1/20”; PLC Rice 1/20 (0.125 % protein), “1/25”; PLC Rice 1/25 (0.1 % protein)
Another dietary consideration for CKD patients is the desirability of reducing phosphorus and potassium intake in addition to protein. At the same time, there is a need to ensure the patient takes in enough nutrients, particularly energy. With PLC Rice products, not only can protein intake be reduced to the desired level, but the products contain 50% less phosphorus and 95% less potassium compared to regular cooked rice while offering the same energy as WR. They meet all of the requirements as a staple food for CKD patients (Figure 7).
Figure 7: Nutrient Content in “Cooked PLC Rice” Series.
We developed exclusive technology for digesting and removing protein from WR using microorganism-derived enzyme product, and technology for processing the Raw LPR thus obtained into a palatable food product. By combining these technologies, we can reliably manufacture delicious, high quality, low protein rice product, which can be eaten repeatedly as staple. In the 20 years from its launch, nearly 100 million PLC Rice products have been sold, with zero complaints relating to health problems arising from their consumption to date. This demonstrates their safety even when eaten repeatedly over a long period.
The PLC Rice series of products, which compare favorably with, and can be substituted for, regular rice, are delicious (high quality) enough to be eaten everyday
CONCLUSION
LPR enhances the quality of mealtimes for CKD patients by increasing their range of food choices. It is therefore, reasonable to say that PLC Rice products offer high added value, as it not only facilitates adhesion to LPD but also add satisfaction and contentment to daily meals, helping to enhance the QoL of patients with CKD.
CONFLICTS OF INTEREST
All authors are employee of Forica Foods Co., Ltd., Uonuma City, Niigata, Japan. All of this study and work has been performed at the Forica Food Co., Ltd., Uonuma City, Niigata, Japan.
ACKNOWLEDGEMENT
The authors appreciate to Dr. Shaw Watanabe (Life Science Promoting Association, Tokyo, Japan) for his great advices on this study. The authors would also like to tahnk all the staff of Institute of Food Research and Product Development Kasetsart University (bangkok, Thailand), especially ms. Ngamjit Lowithun and Mrs. patcharee Tungtrakul, for their helpful advice and support to our research.. A part of this study was supported by Grant from Japan International Cooperation Agency (JICA).












