INTRODUCTION
Breast cancer is the commonest solid tumor affecting females worldwide and the second leading cause of cancer relating mortality.1 The impact on women and their families, the morbidity and the change of millions of lives remains enormous. Cytotoxic drugs or hormonal agents have remained for decades the backbone of treatment. Fortunately, with the recognition of distinct subtypes of breast adenocarcinoma with variable natural course and prognosis, the therapeutic options have broadened with the addition of biological agents, targeted therapies, monoclonal antibodies and recently immunotherapy drugs. The latest have changed the outcome of many solid tumors once seemed fatal, such as melanoma, lung cancer, renal cancer, etc.
We have now data that some of these drugs work in specific cases of breast cancer. In this mini review we will try to put the current knowledge and data of immunotherapy in triple negative breast cancer (TNBC) (Figure 1).
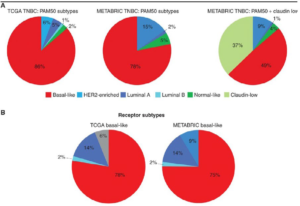
Figure 1. Molecular Subtypes of Breast Cancer in pivotal Genomic Studies2
CONCEPT OF IMMUNOTHERAPY IN BREAST CANCER
One of the recognized hallmarks of cancer growth and progression is its escape from destruction by immune cells. We now know that this might happen due to overexpression of molecules that halt the activity of immune reaction, in other words that work as immune breaks. Such molecules are the PD1 (Programmed Death 1), PD-L1 (Programmed Death-Ligand 1) and CTLA-4 (Cytotoxic T-Lymphocyte Antigen-4) proteins. When the PD-1 receptor protein or its ligand is upregulated in the T-cells or the cancer cells we observe both innate and adaptive immunity inhibition. Likewise, overexpression of the inhibitory CTLA-4 protein in the T-lymphocytes leads to inactivity of immune system allowing further progression of cancer cells.
TNBC represents around 15% of advanced breast cancer patients.2 It is associated with poor prognosis compared to the other forms and its management remains an oncological challenge.
Initially, breast cancer was deemed to be a non-immunogenic cancer with low tumor burden but recent research has suggested that TNBC yields immunogenic features subjective to targeting with the novel immunotherapy agents. HER2 positive and TNBC express higher levels of PD-L1 protein.3 Triple-negative breast cancers (TNBC) as well as the HER2-positive breast cancer are also more likely than the luminal subtypes to harbor higher stromal-infiltrating immune cells (TILs) at diagnosis. There are data showing a linear relationship between stromal TILs and better clinical outcomes.4
EARLY CLINICAL DATA
Early phase clinical studies (Ia/Ib) in metastatic breast cancer patients showed that there is some response from immunotherapy treatment with avelumab (the ZAVELIN Ia/Ib study, ORR 8.6% but 44% in TNBC PD-L1+patients),5 pembrolizumab (KEYNOTE-012 Ib study, ORR 18.5%)6 or atezolizumab (ORR 13% in PD-L1+ TNBC).7 Though numerically the benefit was small, it was somehow encouraging given the fact that immunotherapy in these studies was used as monotherapy in an already heavily pretreated population.
Following those early data, combination of these checkpoint inhibitors with chemotherapy agents was next tested.
Atezolizumab (840 mg every 2-weeks) was combined with nab-paclitaxel (125 mg/m2 on days 1, 8, and 15 every 28-days) in a phase Ib study in 32 metastatic TNBC patients of any PD-L1 status. After a median follow-up over 5-months, the ORR was 38% (1 CR, 11 PRs).8
Pembrolizumab (200 mg i.v. every 3-weeks) was tested with eribulin (1.4 mg/m2 on days 1 and 8 every 21-days) in an phase II trial designed to enroll 107 patients with metastatic TNBC of any PD-L1 status treated with ≤2 prior lines of chemotherapy. The ORR for the combination was 26.4% (3 CRs, 25 PRs in 106 out of 107 evaluable patients). The mOS was 17.7-months and the duration of response (DoR) 8.3-months for the 28 patients with objective response.9
LATE PHASE STUDIES
Following the above encouraging results researchers and industry moved to larger phase III studies.
The KEYNOTE-355 phase III trial is evaluating pembrolizumab with chemotherapy relative to various chemotherapy regimens alone as first-line therapy for incurable TNBC. The study is expected to complete accrual in December 2019.10
Very recently, results from the IMpassion130 phase III study were presented at both the ESMO 2018 Congress and the ASCO 2019 Annual Meeting (31st May-4th June, 2019).11
The IMpassion 130 was a phase III trial that enrolled 902 patients with metastatic triple-negative breast cancer treatment naïved for metastatic disease.12 Patients were randomized between the standard chemotherapy (nab-paclitaxel 125 mg/m2 on days 1, 8, and 15 every 28-days ) plus atezolizumab (840 mg every 2-weeks) and the standard chemotherapy plus placebo. The primary endpoints were progression-free survival (PFS) and overall survival (OS). The patients were followed for a median follow-up time of 12.9-months. The two arms were quite balanced in terms of demographic details and the results of the study are the following.
The chemotherapy/immunotherapy combination arm reduced the risk of disease progression and death by 20% in all patients and 38% in the subgroup overexpressing PD-L1. In the whole study population, the median PFS was 7.2-months with the immunotherapy combination and 5.5-months with chemotherapy/placebo (hazard ratio [HR]=0.80, p=0.0025). Of interest, the PD-L1–positive group showed a median progression-free survival of 7.5-months with the combination and 5-months with chemotherapy alone (HR=0.62, p<0.0001).
Though the PFS data supported the use of immunotherapy in TNBC, the updated overall survival results presented at the 2019 American Society clinical oncology meeting showed that in the whole study population there is no significant clinical benefit in the atezolizumab arm (median OS=21.0 vs 18.7 months; HR=0.86, 95% CI: 0.72-1.02, p=0.078) although the selected PDL1 positive TILs cohort did get survival benefit (median OS=25.0 vs 18.0 months; HR=0.71, 95% CI: 0.54-0.93). Another important observation was that after two years follow-up, over 50% (43-59%) of PD-L1-positive metastatic TNBC patients in the atezolizumab arm were alive, compared to 37% (29-45%) in the control arm.
The objective response rate (a secondary endpoint) was higher in the combination group compared to the standard chemotherapy/placebo group for the whole patients cohort (56% vs 46%) and especially those with PD-L1-positive tumors (59% vs 43%) (Figures 2 and 3).
Figure 2. Updated Survival Data Within the IMpassion 130 study11
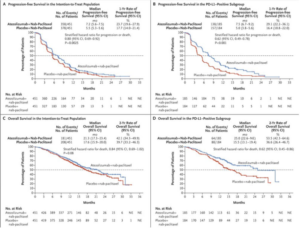
Figure 3. PD-L1 Expression on Tumor Cells (TC) and Tumor Infiltrating Immune Cells (IC) in IMpassion130 Study8
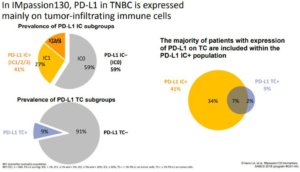
Regarding safety no particular concerns were noted and the side effects related to immune therapy were rare, the most common being hypothyroidism, that occurred in 17.3% of patients receiving immunotherapy and 4.3% receiving chemotherapy alone. Most side effects were due to chemotherapy and occurred at a similar rate in both treatment groups, although there was a minor increase in nausea and cough in the combination group. Similarly, the quality of life was satisfactory in both groups.11
CONCLUSION
This mini review shows that the field of immunotherapy in breast cancer is still evolving. There is no outstanding benefit in the whole breast cancer patients, even in the TNBC subtype. Biomarkers such as PD-L1 expression or TILs seem to play some role but more data is needed. There is no doubt that the much expected results from the ongoing phase III studies and exploratory studies will shed more light to the many questions we still have. But we feel confidence that with more research will find the settings where immunotherapy benefits breast cancer patients similarly to other solid tumors.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








