INTRODUCTION
Diverticular disease of the colon is commonly encountered in western countries, affecting up to half the population over the age of 60.1,2 Only 10-25% of patients with diverticulosis develop diverticulitis and go on to present with acute abdominal symptoms and/or complications such as perforation, abscess or fistula formation or infection.1 Colorectal cancer is another common pathological entity in the West, as the third most commonly diagnosed cancer and the third leading cause of cancer death in both men and women in the US.3 Cases of colon cancer arising in colonic diverticula have been reported, but are still quite rare, and consensus guidelines on the diagnostic workup have yet to be established.4,5,6,7 We report the case of a 77-year-old male patient who presented for the first time with perforated diverticulitis and abscess formation. Histopathological analysis of the sigmoid colectomy specimen revealed a mucinous adenocarcinoma that eluded the computarized tomography (CT) imaging. In this article, we report in detail the clinical scenario of this patient and review the literature for comparable cases and the current diagnostic practices.
CASE PRESENTATION
A 77-year-old male patient was referred to our medical center from an outside hospital after presenting for the first time with acute onset of abdominal pain localized to the left lower quadrant, nausea, vomiting, fever, chills, malaise, anorexia and diarrhea. The laboratory results were found to be unremarkable except for a white blood cell count of 16,300 /µL and creatinine level of 1.7 mg/dL. Electrocardiogram was unremarkable and arterial oxygen saturation was 95%. A CT scan with intravenous contrast revealed extensive colonic diverticulosis, thickening of the wall of the sigmoid colon, consistent with sigmoid diverticulitis and extraluminal air compatible with a micro-perforation. In addition, a 4.9×4.6 cm abscess was identified adjacent to the colon. No bowel obstruction or enlarged (abdominal or pelvic) lymph nodes were recognized by. The patient’s past medical history included bladder cancer with cystectomy and neobladder reconstruction, appendectomy, and prostatectomy 15 years earlier. The histopathological type and staging of the bladder cancer is not known. Moreover, the patient also had a history of coronary artery disease with coronary angioplasty and stent placement, atrial fibrillation with placement of an inferior vena cava filter, chronic obstructive pulmonary disease, diabetes mellitus, hypertension and deep venous thrombosis. He was also a heavy smoker for 24 years who quit at the age of 40. There was no history of alcohol use or known allergies. Due to the persistence of abdominal pain and severe anorexia, the patient underwent exploratory laparotomy, during which diverticulitis was confirmed. A partial (sigmoid) colectomy was performed. The main resection specimen consisted of a 9.5 cm long segment of sigmoid colon with attached pericolic fat, as well as pelvic abscess contents, containing gelatinous material admixed with firm pink tan tissue. Upon opening the bowel, the congested mucosa showed many diverticula ranging in depth from 0.5 to 1.5 cm associated with necrotic tissues (Figure 1A). Histopathological examination revealed diverticulitis (Figure 1B) and pericolonic adhesions, but no tumor. The pelvic abscess contents showed mucinous adenocarcinoma with positive staining for CDX2 and CK20 and negative staining for CK7 consistent with a primary colonic adenocarcinoma (Figure 2).
Figure 1. Panel A shows a gross picture of a cross section of the sigmoid colon with an arrow indicating a diverticulum and an
asterisk indicating the site of perforation. Panel B shows 40x magnification of an H&E stained
section showing a diverticulum with surrounding acute and chronic inflammatory infiltrate.
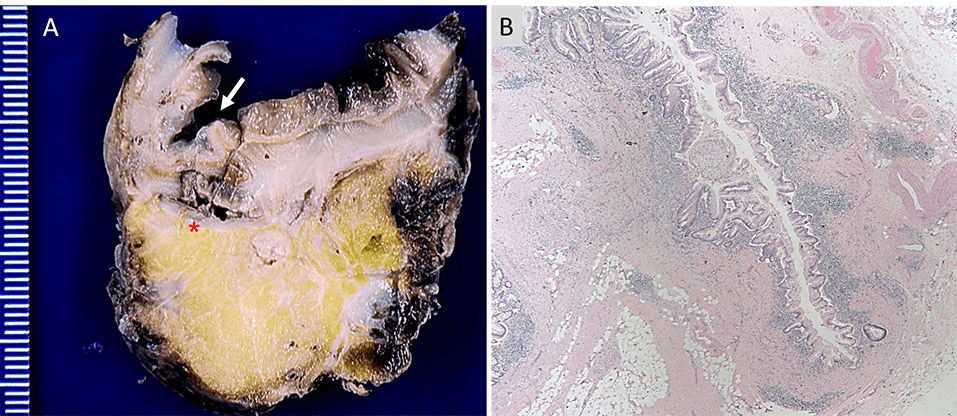
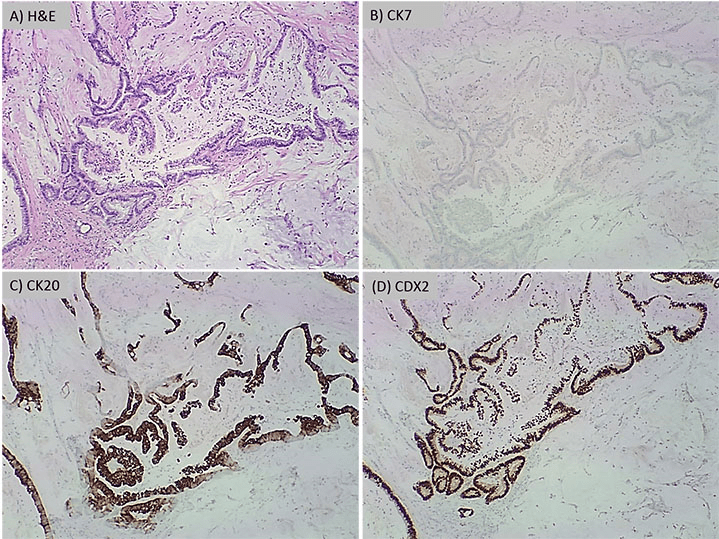
Figure 2: Panel A is H&E at 40x showing mucinous adenocarcinoma. The immunohistochemical profile shows
the tumor cells with negative staining for CK7 (B) and positive staining for CK20 (C) and CDX2 (D)
On post-operative day 4, due to worsening clinical symptoms of perforated sigmoid diverticulitis and newly developed peritonitis, a subsequent distal sigmoid colectomy was performed. Upon opening the bowel, the hemorrhagic colonic mucosa showed multiple perforated diverticula surrounded with necrotic tissue, but no visible masses were identified. Histopathological examination revealed a moderately-differentiated mucinous adenocarcinoma (Figure 3) measuring 0.9 cm in greatest dimension associated with the perforation site in the distal sigmoid colon. The tumor had moderate intramural and peritumor lymphocytic responses. Immunohistochemical analysis again showed positive immunostaining of the tumor cells for CK20 and CDX2 and negative immunostaining for CK7, supporting the diagnosis of a primary colonic adenocarcinoma. The tumor was found to invade through the muscularis propria into pericolonic soft tissue and visceral peritoneum. The proximal, distal and radial margins were not involved and there was no perineural invasion, lymph-vascular invasion, or regional lymph node metastases. In addition, a low grade mucinous neoplasm was seen in the vicinity of the mucinous adenocarcinoma (Figure 4). Additional pathological findings included ischemic necrosis, multiple diverticula, acute and chronic inflammation, abscess formation and acute organizing serositis. As such, the tumor was classified according to the American Joint Committee on Cancer (AJCC) Pathological Staging 7th edition as (pT4a, pN0). Additionally, the specimen was submitted for KRAS mutation analysis, revealing that KRAS codon 12 mutation (c.35G>T) was present. Unfortunately, the patient died within six days of presentation to our hospital from septicemia as a complication of perforated diverticulitis.
Figure 3. H&E at 100x of mucinous adenocarcinoma arising within a diverticulum
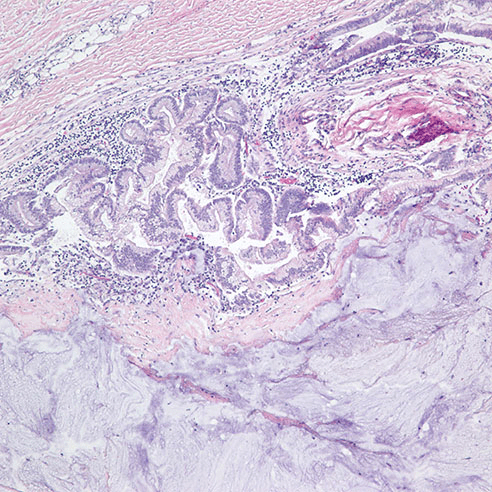
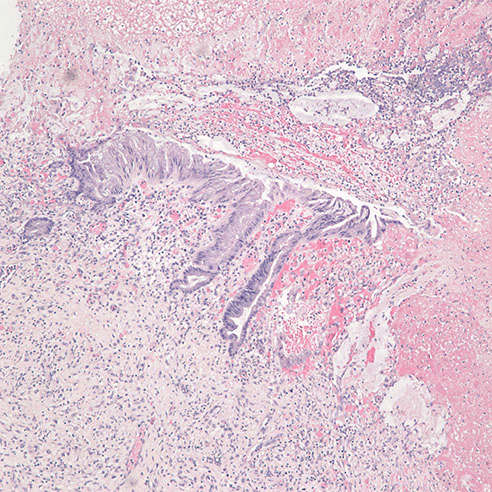
Figure 4. H&E at 40x, low grade mucinous neoplasm near the diverticulum
DISCUSSION AND LITERATURE REVIEW
The incidence of diverticulosis and diverticulitis has been rising in the western world due to dietary habits and prolonged life expectancy such that diverticulosis now affects 48% of the developed world population over the age of 50.1,2 Diverticulitis typically presents as “acute abdomen” with acute abdominal pain, fever, diarrhea or constipation, nausea and vomiting.1,8,9 Aging and western diet are also risk factors for increased incidence of colorectal cancer which is the third most common cancer type in the US. The presentation of colorectal cancers can vary considerably, from being entirely asymptomatic to bleeding per rectum, melena or constipation up to frank intestinal obstruction. Colorectal cancers arising in colonic diverticulitis have been reported but remain very rare with few reported cases.4,5,6,7,10,11,12,13,14,15,16,17 This co-occurrence is proposed to represent a diagnostically and therapeutically significant correlation8 rather than a causal relationship18 with only poorly defined guidelines existing to guide diagnosis and eventual management of the coexisting conditions. Interestingly, benign polyps are also reported to arise in association with colonic diverticula.19,20 From a prognostic point of view, colorectal carcinoma arising from diverticula may have a higher likelihood of extramural spread to neighboring organs due to the deficiency of the muscular layer in diverticula. In support of this, Yagi et al15 reported a case of a sigmoid colon adenocarcinoma arising from a colonic diverticulum and invading the urinary blabber via a colovesical fistula. Importantly, attempts at endoscopic mucosal resection can be complicated by iatrogenic perforation requiring subsequent hemicolectomy.13 Rendering the proper diagnosis is one of the challenges for concomitant diverticulitis and adenocarcinoma given the different diagnostic techniques for each clinical entity. The diagnostic modality of choice for colorectal adenocarcinoma is colonoscopy which may fail in identifying submucosal lesions especially in the absence of frank mucosal involvement. On the other hand, CT scan is the standard diagnostic modality of choice for diverticulitis, but its findings can be non-specific. Indeed, thickening of the intestinal wall, localized inflammatory reaction, enlarged pericolic lymph nodes, are all radiological findings commonly found in colorectal carcinomas as well.21
Likewise, barium enema, positron emission tomography-computed tomography and magnetic resonance imaging may fail in distinguishing inflammatory from malignant conditions. In addition to radiological ambiguity, some diverticulitis cases have been reported to present typically as reduced stool caliber due to considerable stenosis rather than the conventional acute abdomen presentation.22 Therefore, distinguishing diverticulitis from carcinoma-induced stricture can be challenging without histopathological examination. Studies show that the incidence of colorectal cancer at the site of diverticulitis one year after the episode is 44-fold higher compared to the age-matched general population.23 Similarly, adenomas are reported to have a significantly higher incidence in association with diverticular disease.24 Therefore, practice guidelines recommend performing a colonoscopy after the resolution of an episode of diverticulitis regardless of the clinical or radiological presentation, in order to detect unsuspected etiologies, particularly neoplastic conditions.25 However, because the incidence of carcinoma is significantly lower following an uncomplicated diverticulitis (0.3%) than following a complicated one (7.6%), it has been proposed that endoscopic evaluation may be skipped in patients with uncomplicated diverticulitis.26 We propose that in a patient with a long history of diverticulitis, multiple colorectal cancer high risk factors, and any discrepancy between radiology, clinical and pathological findings, a thorough investigation is warranted.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









