INTRODUCTION
Human hookworm infection cause one of the world’s most debilitating soil-transmitted tropical diseases, with an estimated 740 million cases in tropical and subtropical areas.1 Yet, it is often neglected. Hookworm infection predominantly affects low socio-economic populations, causing chronic iron deficiency anemia, reduced work capacity in adults, and pregnancy complications in females, and impaired growth and development in infants. The control of hookworm relies heavily on the use of anthelmintic drugs, specifically albendazole and mebendazole. However, rapid reinfection rates in mass administration programs and increasing concerns around resistance to these drugs have heightened the need for an anti-hookworm vaccine.2
Necator americanus aspartic protease-1 (Na-APR-1) is a cathepsin D aspartic protease that has been targeted for subunit vaccine development, as it is responsible for degrading host hemoglobin into a food source inside the gut of the adult hookworm, and therefore is crucial for hookworm nutrition and survival. However, production of APR-1 protein is complicated especially purification process due to protein hydrophobic nature.3,4,5 More importantly, APR-1 protein generates antibody responses against several redundant epitopes (e.g. S107L), which may overwhelm desired neutralizing antibody responses.3,4 Therefore, we identified a peptide derived from the protein, called p3 (TSKIAGPKAQVEAIQKYIGAEL), which can induce antibodies that neutralize the enzymatic activity of the Na-APR-1 protease following intraperitoneal immunization,4 and which are homologues in different human- and animal hookworm species.3 The p3 peptide also stimulates neutralizing antibodies when orally delivered in lipid-based delivery systems.6 However, oral vaccination with the p3 peptide requires multiple, large doses of the vaccine, which presents a significant downside to this delivery pathway. Nasal vaccine delivery was shown to stimulate an immune response to other pathogens with lower dose and number of immunizations.7,8,9 As with the oral route, the nasal delivery pathway offers many advantages for vaccine distribution, including the possibility of self-administration across the population, including for children and the elderly, elimination of needle stick injury risk, and the ability to induce both mucosal and systemic immunity.10 However, intranasal immunization requires a mucosal delivery system to trigger enhanced immune responses. Intranasal immunization has shown to successfully stimulate protective immune responses. Recently, a nanoparticle vaccine constituted of extract from Toxoplasma gondii was shown to confer a high-level of protection against toxoplasmosis in sheep after two intranasal immunizations.11
Conjugation of synthetic lipids to peptides can produce vaccine constructs that stimulate an immune response without the requirement of an external adjuvant, as well as facilitate the incorporation of antigen into liposome-based delivery systems. A lipid core peptide (LCP) system combines the antigen, a branching moiety and lipoamino acids into a single molecule. It has previously been demonstrated that when attached to the previous mentioned hookworm p3 peptide, the LCP system can assist to stimulate an immune response against hookworm infection.4 As an additional construct, a similarly structured lipid, Pam2Cys, has also shown potential for mediating direct adjuvants activity via Toll-Like Receptor 2 when attached to antigens against viral and bacterial infections compared to non-lipidated peptides.12
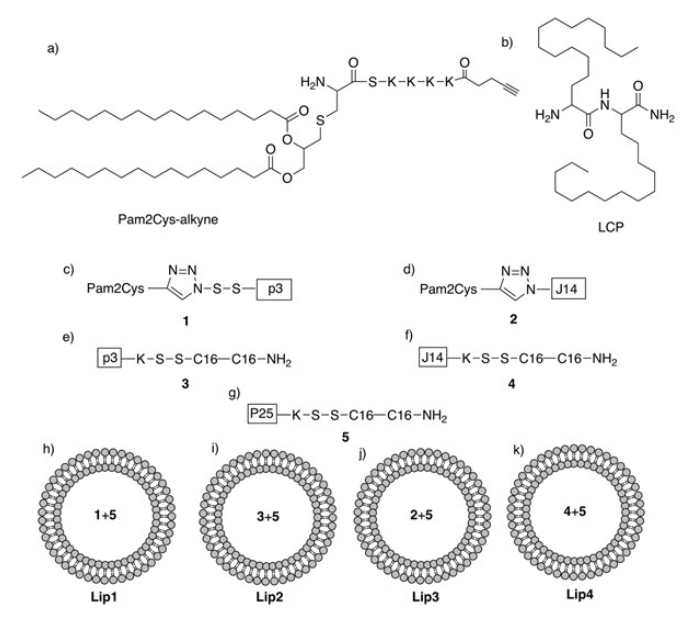
In this study, we focused on the development of a peptide-based intranasal vaccine against hookworm infection. The vaccine candidates were composed of the p3 B-cell epitope derived from Na-APR-1,4 the T-helper epitope P25,12,13 and the lipid adjuvating moiety Pam2Cys or LCP, encapsulated into a liposome or self-assembled into nanoparticles. Lipid-based vaccine candidates were formulated and tested for vaccine efficacy in a rodent hookworm challenge model (Figure 1).
Figure 1. Schematic Representation of the Vaccine Candidates. Lipids: (a) Pam2CysSKKKK-alkyne (Pam2Cys-alkyne), (b) LCP (C16-C16); Lipopeptides: (c) 1 (Pam2Cys-p3), (d) 2 (Pam2Cys-J14), (e) 3 (p3-LCP), (f) 4 (J14-LCP), (g) 5 (P25-LCP); Liposomal Vaccine Candidates: (h) Lip1, (i) Lip2, (j) Lip3, (k) Lip4
EXPERIMENTAL MATERIALS
All chemical materials used in this study were analytical grade, unless otherwise stated. Protected Fmoc amino acids and 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-Oxide Hexafluorophosphate (HATU) were purchased from Mimotopes (Melbourne, Australia). Rink-amide p-methylben zhydrylamine (MBHA) resin was purchased from Novabiochem (Hohenbrunn, Germany). Dichloromethane (DCM), diethyl ether, piperidine, trifluoroacetic acid (TFA), N,N’-dimethylformamide (DMF), HPLC grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). 4-Pentynoic acid was purchased from Alfa Aesar (MA, USA). N,N’-diisopropylethylamine (DIPEA), triisopropylsilane (TIS), acetic anhydride, 2-azidoacetic acid, copper wire, cholera toxin B subunit (CTB), and antimouse IgG conjugated to horseradish peroxidase were purchased from Sigma-Aldrich (St Louis, USA). Dipalmitoylphosphatidylcholine (DPPC), cholesterol, dimethyldioctadecylammonium bromide (DDAB), an Avanti mini extruder, PC membranes and filter supports were bought from Avantis Polar, Inc. (AL, USA); Auspep Pty. Ltd., VIC, Australia. All other reagents were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). J14-azide and 4 were synthesized as previously reported.14,15
Synthesis of Lipid Moieties
Synthesis of Pam2Cys-alkyne: Pam2Cys-alkyne was synthesized on rink amide MBHA resin using 9-fluorenylmethylcarbamate solid-phase peptide synthesis (Fmoc-statistical package for the social sciences (SPPS)), as previously reported.16
Synthesis of Fmoc-Dhc-OH
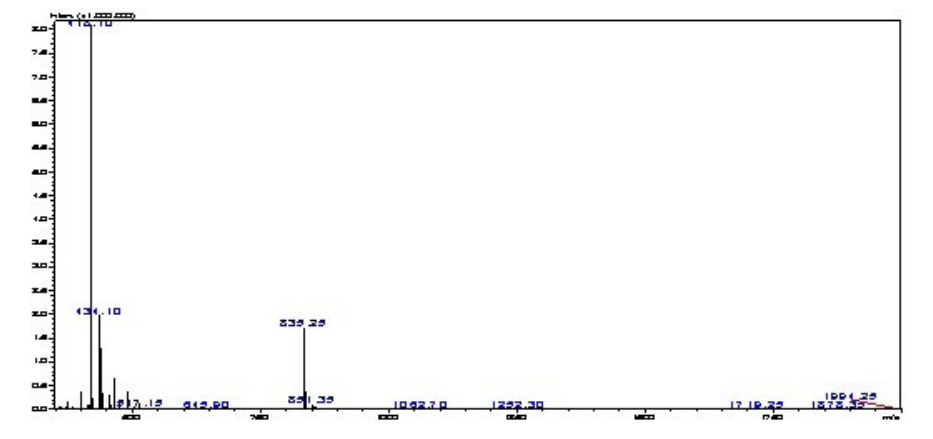
A mixture of cysteine hydrochloride (0.73 g, 6 mmoL), 3-bromopropan-1,2-diol (1.4 g, 0.79 mL, 9 mmol), trimethylamine (2 g, 2.7 mL, 19 mmol) and H2 O (5 mL) was kept in a cupboard for 3 days, covered with aluminum foil. The mixture was evaporated, washed with acetone (3×15 mL), and dried to give Dhc-OH (0.8 g, 3.5 mmol, 67%). Dhc-OH was dissolved in 9% sodium carbonate (10 mL), combined with fluorenylmethoxycarbonylN-hydrosuccinimide (1.15 g, 3.5 mmoL) in acetonitrile (CH3 CN), then stirred at room temperature for 2-hours. Water was added, and the solution was acidified to a potential of hydrogen (pH) of 2 with concentrated hydrochloric acid (37%). Extraction was performed using ethyl acetate, water and brine in triplicates, dried over anhydrous magnesium sulfate (MgSO4), and evaporated under vacuum. Purification of the crude product was performed using an RP-HPLC C18 column with a 25-45% solvent B gradient over 20 min. tR=25.1 min, purity >95%. Yield: 13%. ESI-MS: m/z 418.1 (calculated 418.5) [M+1H]1+; 835.3 (calculated 836.0) [M+2H]2+; 1252.3 (calculated 1253.4) [M+3H]3+; MW 417 (Figure 2).
Figure 2. Schematic of the Synthesis of Fmoc-Dhc-OH
Synthesis of Pam2Cys-alkyne
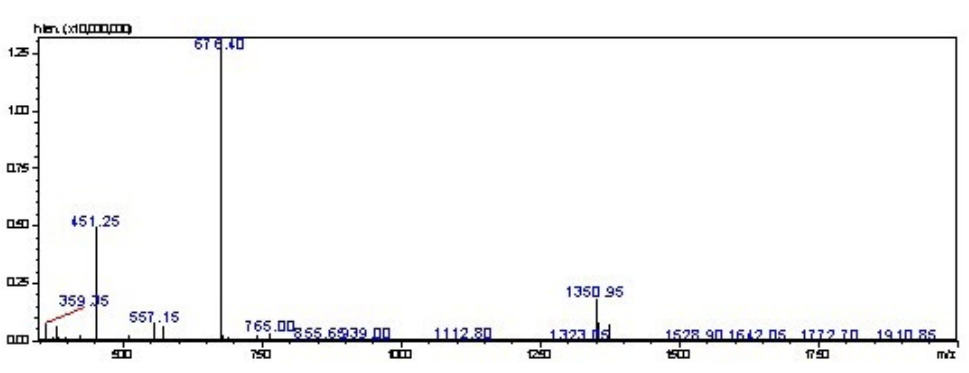
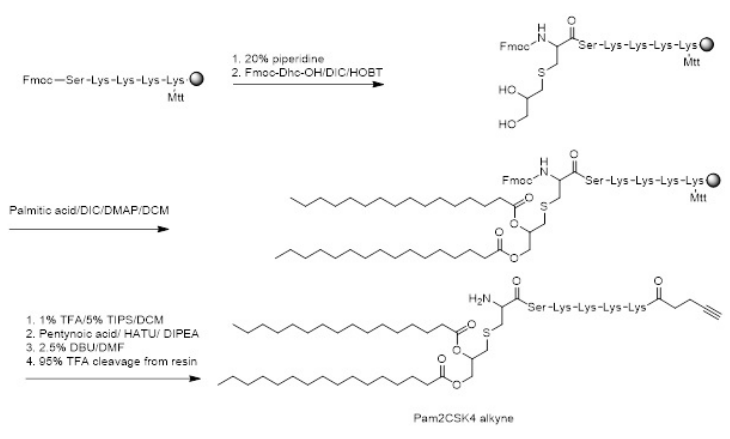
A penta-peptide linker (SKKKK) was synthesized on rink amide MBHA (substitution ratio: 0.79 mmoL/g, 0.1 mmol scale, 0.125 g) resin using Fmoc-SPPS. The resin was split into 0.05 mmoL scale for the continuation of Pam2Cys (Pam2Cys-SKKKK) synthesis. Fmoc-Dhc-OH (3 equiv) was dissolved in N’, N’- diisopropylcarbodiimide (DIC) (3 equiv) and hydroxybenzotriazole (HOBt) (3 equiv) in dimethylformamide (DMF) (2 mL) at 0 °C for 5 min. The activated Fmoc-Dhc-OH was coupled for 4-hours, followed by washing with DMF and dichloromethane (DCM). The S-glycerol-cysteine hydroxyl groups were palmitoylated with palmitic acid (20 equiv) via activation with DIC (25 equiv) and 4-dimethylaminopyridine (DMAP) (2 equiv) in DCM. Twohour and overnight couplings at room temperature were used to complete the reaction. The reaction was then washed with DCM. The ε-Mtt protecting group on the C-terminal lysine was removed using a mixture of TIS (5%) and TFA (1%) in DCM 20 times, for 5 min each. After deprotection, the reaction was washed with DCM and DMF. 4-pentynoic acid was coupled to the free lysine sidechain using HATU (4 equiv) and DIPEA (5.2 equiv) for 2-hour couplings at room temperature. The Fmoc group present on the Fmoc-DhcOH was removed using 1,8-diazabicycloundec-7-ene (DBU) (2.5%) in DMF, three times, for 5 min each. After completion, the resin was washed with DMF, DCM and methanol and dried overnight under vacuum. Purification of the crude product was performed using RP-HPLC on a C4 column with a 40-80% solvent B gradient over 60 min. tR=32.6 min, purity >98%. Yield: 22%. ESI-MS: m/z 676.5 (calculated 676.5) [M+2H]2+; 451.2 (calculated 451.3) [M+3H]3+; MW 1350.9 (Figure 3).
Figure 3. Schematic Showing the Synthesis of Pam2Cys-alkyne
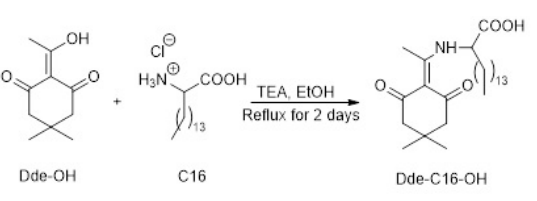
Synthesis of 2-((1-(4,4-dimethyl-2,6-dioxocyclohexylidene) ethyl) amino) hexadecenoic acid (Dde-C16-OH)
Synthesis of Dde-C16-OH was completed according to the standard protocol.17 2-acetyldimedone (Dde) and 2-aminohexadecanoic acid (C16) lipoamino acids were synthesized separately, then conjugated together (as detailed below) for use in the synthesis of the lipopeptides.
Synthesis of 2-Acetyldimedone (Dde)
In a 500 mL round bottom flask, DCM (200 mL) was added to dimedone (23 g, 0.16 mol) and the flask was covered in foil to protect the contents from light. Dicyclohexylcarbodiimide (DCC, 33.0128 g, 0.16 mol), DMAP (19.5472 g, 0.16 mol) and glacial acetic acid (9.38 mL, 0.16 mol) were then added to the suspension. The reaction mixture was stirred for 16 hours at room temperature. Thin layer chromatography (TLC) was used to monitor the progress of the reaction. Upon completion, the reaction was filtered and the solvent evaporated. Extraction was performed using ethyl acetate (300 mL) and 5% HCl (50 mL), both in triplicate. The organic layer was dried over anhydrous magnesium sulphate and then dissolved in hexane and purified using silica-gel to produce a powdered product (Dde-OH). Yield: 80.6%; TLC: Rf (Hex:EtOAc, 3:2)=0.76; 1H NMR (300 MHz, CDCl3): δ 2.58 (s, 3H, CH3), 2.51 (s, 2H, CH2), 2.33 (s, 2H, CH2), 1.05 (s, 6H, CH3).
Synthesis of 2-aminohexadecanoic Acid (C16) Lipoamino Acid
In a 500 mL round bottom flask, sodium (2.3 g, 0.1 mol) was dissolved in ethanol (79 mL). Diethylacetomidomalonate (21.7 g, 0.1 mol) and 1-bromotetradecane (41.7 mL, 0.14 mol) were added and refluxed for 24-hours. The reaction mixture was added to crushed ice and filtered through a Buchner funnel. The compound was dried to a powder, then placed in a 1 L round bottom flask along with DMF (14 mL) and 38% HCl (120 mL), and refluxed for 3-days. Ammonia was added carefully to the reaction mixture after reflux, along with crushed ice. The mixture was filtered, and the resulting product was washed with water and acetone. The product was left to dry until powdery. Yield: 79.61%; TLC:Rf (CHCl3 :MeOH:AcOH, 9:0.8:0.2)=0.4; 1H NMR: δ=4.37 (dd, 1H, α-CH), 2.51 (s, 3H, C(NH)CH3 ), 2.40 (s, 4H, 2CH2 CO), 2.05-1.91 (m, 2H, β-CH2 ), 1.45-1.22 (m, 24H, 12CH2 ), 1.02 (s, 6H, C(CH3 ) 2 ), 0.86 (t, 3H, CH3 ).
Synthesis of LCP-2-((1-(4,4-dimethyl-2,6-dioxocyclohexylidene) ethyl)amino) hexadecanoic acid (Dde-C16-OH)
Ethanol (200 mL), Dde-OH (5.78 g, 0.0317 mol), C16-OH (9.7 g), and trimethylamine (TEA) (9.6 g, 12.53 mL, density of TEA=0.726 g/ml) were added to a 500 mL round bottom flask and refluxed for 2 days (Figure 4). The reaction was monitored using TLC. Upon completion, the reaction was cooled and the solvent evaporated. Ethyl acetate (300 mL) was added to the reaction mixture and the product was washed with 5% HCL (100 mL) and brine (100 mL). The organic layer was dried over anhydrous magnesium sulphate and then dissolved in hexane and purified using silica-gel. Solvent from the pure fractions was evaporated and the product was dried to a powder. The resulting yellow crystallized solid was washed with cold acetonitrile and filtered until white in color. The resulting product (Dde-C16-OH) was dried into a powder. Yield: 36.9%; Rf (Hex:EtOAc, 3:2)=0.76; 1 H NMR (300 MHz, CDCl3 ): δ 6.68 (s, 1H, NH), 4.37 (q, J=6.6 Hz, 1H, CH), 2.51 (s, 3H, CH3 ), 2.39 (s, 4H, CH2 ), 2.06-1.83 (m, 2H, CH2 ), 1.49-1.36 (m, 2H, CH2 ), 1.34- 1.17 (m, 22H, CH2 ), 1.01 (s, 6H, CH3 ), 0.86 (t, J=6.7 Hz, 3H, CH3 ).
Figure 4. Schematic of the Synthesis of Dde-C16-OH
Synthesis of Peptides and Lipopeptides
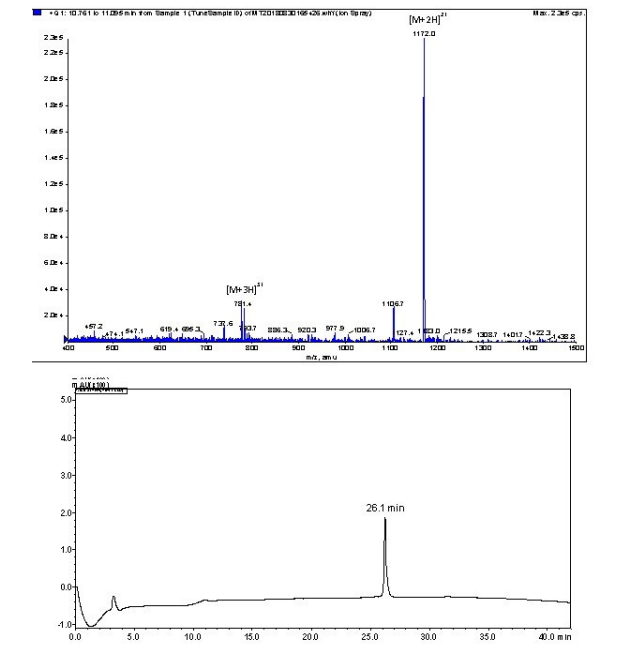
P3: Peptide p3 was synthesized on rink amide MBHA resin using Fmoc-SPPS, as previously reported.17 In summary, the resin (substitution ratio: 0.79 mmoL/g, 0.1 mmol scale, 0.127 g) was swelled overnight in DMF. Synthesis was carried out using microwave assisted Fmoc-SPPS and an SPS mode CEM Discovery reactor. Deprotection of Fmoc was performed twice using 20% piperidine/DMF (2 min and 5 min) at 70 °C. The amino acids were activated using 0.5 M HATU (0.8 mL, 4 equiv) and N,Ndiisopropylethylamine (DIPEA) (0.91 mL, 5.2 equiv) in DMF for 5 and 10 min couplings. Unreacted resin was acetylated with acetic anhydride, DIPEA, and DMF (5:5:90) at 70 °C, twice (5 min and 10 min). All other amino acids were then coupled to the resin using the same procedure. The procedure was repeated until the desired sequence was obtained. When the synthesis was complete, the resin was washed with DMF, DCM, and MeOH, then dried under vacuum. The peptide was cleaved using 95% trifluoroacetic acid (TFA), 2.5% triisopropyl silane (TIS) and 2.5% water (H2 O). TFA was removed under reduced pressure and the lipopeptide was washed with cold diethyl ether. The lipopeptide was dissolved in acetonitrile:water:TFA (50:50:0.1) and dried to produce an amorphous powder. The peptides were purified using preparative RPHPLC on a C18 Vydac column with a solvent gradient of 45-65% solvent B over 30 min. Analytical analysis was preformed using a Shimadzu instrument (C18 column): tR=26.1 min, purity >95%. Yield: 35%. ESI-MS: m/z 1172.0 (calculated 1171.8) [M+2H]2+; 781.4 (calculated 781.5) [M+3H]3+. MW=2341.7.
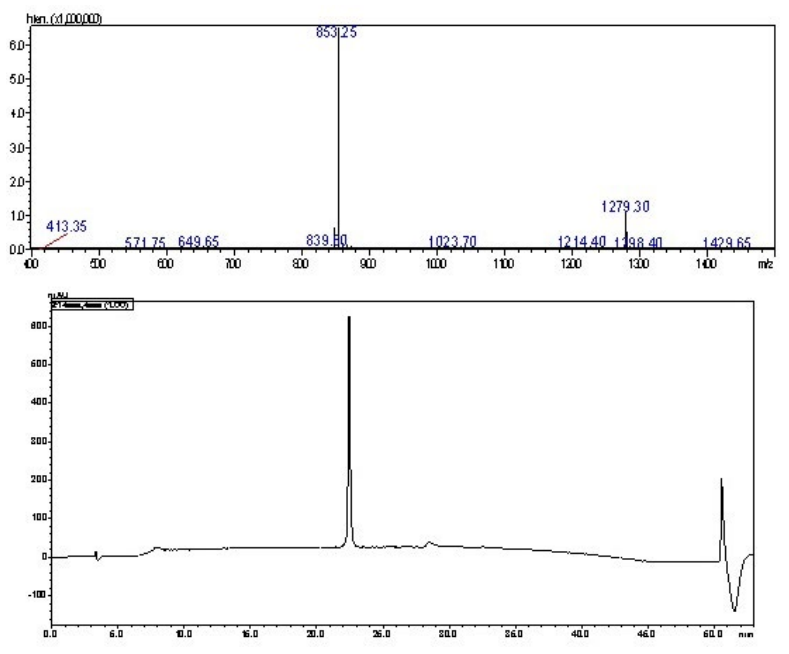
Azide-p3: Azide-p3 (N3 CH2 C(O)-SS-p3) was synthesized as above (substitution ratio: 0.79 mmoL/g, 0.1 mmoL scale, 0.127 g), using HATU/DIPEA Fmoc-chemistry. The attachment of azidoacetic acid (0.32 mL, 4.2 equiv) was achieved using HATU (0.6 mL, 3 equiv)/DIPEA (0.91 mL, 5.2 equiv) at room temperature for 4-hours in the dark. Upon synthesis completion, the resin was washed with DMF, DCM, and MeOH, then dried under vacuum. The peptide was cleaved using 95% trifluoroacetic acid (TFA), 2.5% triisopropyl silane (TIS) and 2.5% water (H2 O). TFA was removed under reduced pressure and the lipopeptide was washed with cold diethyl ether. The peptide was dissolved using water: acetonitrile: TFA (50:50:0.1) and freeze-dried into amorphous powder form. The peptide was purified using preparative RP-HPLC on a C18 Vydac column with a solvent gradient of 40-60% solvent B over 30 min. Analytical analysis was performed using a Shimadzu instrument (C18 column): tR=23.5 min, purity >98%. Yield: 22%. ESI-MS m/z 1279.8 (calculated 1279.5) [M+2H]2+; 853.5 (calculated 853.3) [M+3H]3+; MW=2556.9.
P3-P25: Peptide p3-P25 was synthesized using Fmoc-SPPS as previously reported6 (substitution ratio: 0.79 mmoL/g, 0.1 mmoL scale, 0.127 g) and purified using preparative RP-HPLC on a C18 Vydac column with a 45-65% solvent B gradient over 30 min. tR=26 min, purity >95%. Yield: 32%. ESI-MS: m/z 1423.6 (calculated 1423.3) [M+3H]3+; 1067.8 (calculated 1067.7) [M+4H]4+; 854.3 (calculated 854.4) [M+5H]5+; 701.8 (calculated 701.8) [M+6H]6+. MW=4267.1.
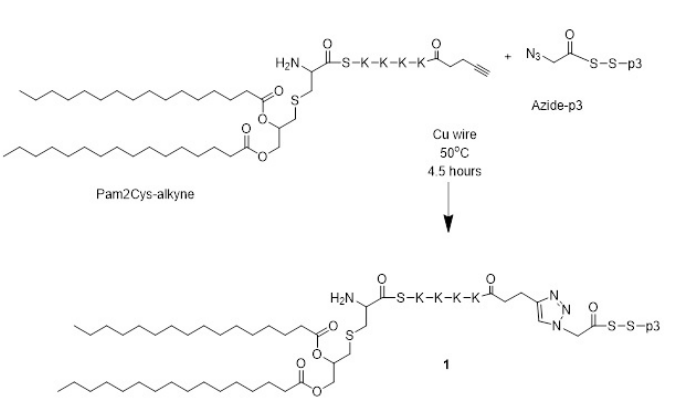
Synthesis of Lipopeptide 1 Using Copper-Catalyzed Alkyne-Azide 1,3-dipolar Cycloaddition (CuAAC) Click Reactions
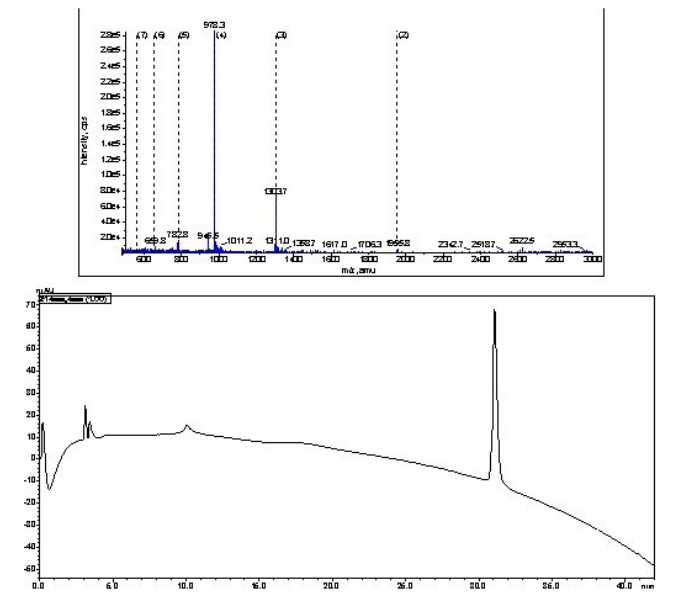
Azide-p3 (4.74 mg, 1.7×10-4 mmoL, 1 equiv) and Pam2Cysalkyne (5 mg, 2.7×10-4 mmoL, 1.6 equiv) were dissolved in DMF (1 mL) and DMSO (0.5 mL). Copper wire (70 mg) was added to the solution (Figure 5). The air was removed from the flask via nitrogen bubbling. The reaction was protected from light using aluminum foil and stirred at 50 °C under nitrogen. The reaction was monitored by analytical HPLC and ESI-MS until all peptide was used (4.5-hours). The reaction was purified using semipreparative HPLC on a C4 column with a 40-80% solvent B gradient over 60 min. Analytical analysis was performed using a Shimadzu instrument (C4 column): tR=31.2 min, purity >98%. Yield: 29%. ESI-MS: m/z 1955.8 (calculated 1954.6) [M+2H]2+; 1303.7 (calculated 1303.6) [M+3H]3+; 978.3 (calculated 977.9) [M+4H]4+; 782.2 (calculated 782.6) [M+5H]5+; MW=3907.9.
Figure 5. Schematic of the Click Reaction between Pam2Cys-alkyne and azide-p3
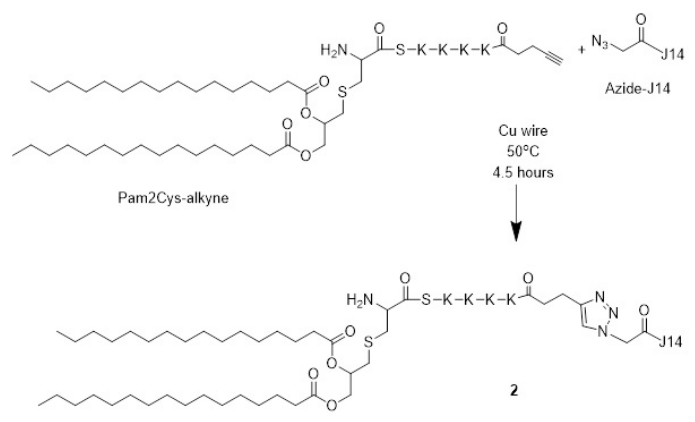
Synthesis of Lipopeptide 2 Using CuAAC Click Reactions
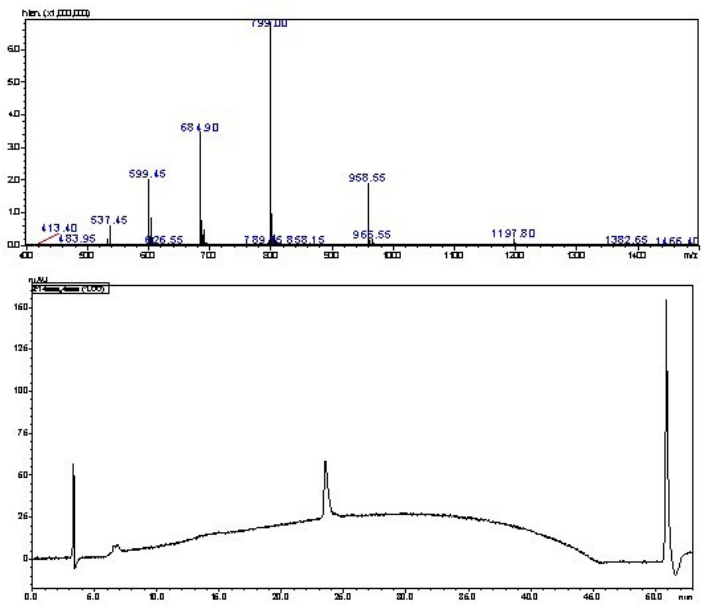
Azide-J14 (N3CH2C(O)-J14) (9.8 mg, 2.86 mmoL, 1 equiv) and Pam2Cys-alkyne (3.9 mg, 2.2 mmoL, 1 equiv) were dissolved in DMF (1.3 mL) and DMSO (0.2 mL) with cooper wire (72 mg) and left for a reaction time of 4.5-hours (Figure 6). The synthesis continued in the same manner as above. The reaction was purified using semi-preparative HPLC on a C4 column with a 40-70% solvent B gradient over 60 min. Analytical analysis was preformed using a Shimadzu instrument (C4 column): tR=23.3 min, purity >98%. Yield: 15%. ESI-MS: m/z 1197.8 (calculated 1197.3) [M+4H]4+; 958.6 (calculated 958.0) [M+5H]5+; 799.0 (calculated 798.5) [M+6H]6+; 684.9 (calculated 684.6) [M+7H]7+; 599.5 (calculated 599.1) [M+8H]8+. MW=4785.8.
Figure 6. Schematic of the Click Reaction between Pam2Cys-alkyne and azide-J14
Lipopeptide 3
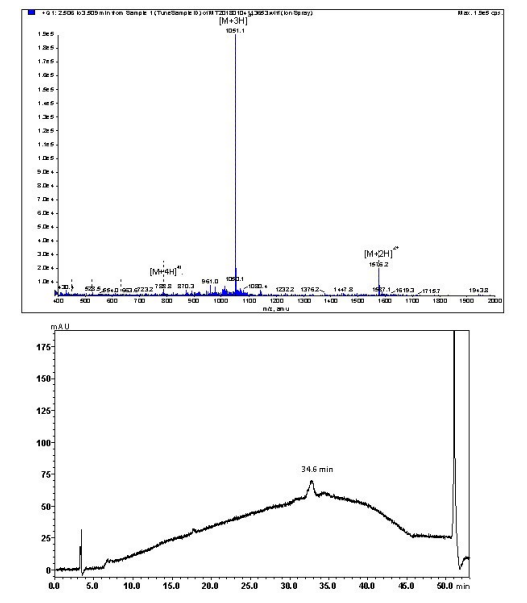
Lipopeptide 3 was synthesized using Fmoc-SPPS on a 0.2 mmol scale (substitution ratio: 0.79 mmoL/g, 0.253 g) using rink amide MBHA resin.6 Dde-C16-OH (0.37 g) was activated using HATU (1.6 mL, 4.0 equiv) and DIPEA (0.91 mL, 5.2 equiv) in DMF for 10 min. The activated solution was added to the resin and coupling time was 1-hour at 70 °C. Dde was removed, after the coupling of Dde-C16-OH, using a solution containing 5% hydrazine/DMF over three periods at 70 °C. The first and second periods were for 10 min each and the third period was for 20 min. Unreacted resin was acetylated with acetic anhydride, as above. Subsequently, the second Dde-C16-OH lipoamino acid and remainder amino acids were coupled to the resin following the same procedure as above. Lipopeptide 3 was purified using RP-HPLC on a C4 Vydac column with a solvent gradient of 45-65% solvent B over 30 min. Analytical analysis was preformed using a Shimadzu instrument (C4 column): tR=30.6 min, purity >95%. Yield: 25%. ESI-MS: m/z 1576.2 (calculated 1576.4) [M+2H]2+; 1051.1 (calculated 1051.3) [M+3H]3+; 788.8 (calculated 788.7) [M+4H]4+. MW=3150.9.
Lipopeptide 4
Lipopeptide 4 was synthesized as previously reported.15 Briefly, lipopeptide 4 was synthesized by tert-butyloxycarbonyl (Boc) SPPS, on a 0.2 mmoL scale using MBHA resin. Coupling reagents used to activate and couple the amino acids were the same as those in the Fmoc synthesis. Microwave-assisted Boc SPPS was performed by using a SPS mode CEM Discovery reactor. Each amino acid was coupled twice, 1×5 min and 1×10 min at 70 °C with DMF washes in between. Removal of the Boc group was carried out using TFA, 2×1 min with stirring at room temperature. Hydrofluoric acid (HF) was used to cleave the peptides from the resin. HF cleavage (10 mL HF/g resin) was achieved at -8 °C in the presence of 5% (v/v) p-thiocresol and 5% (v/v) p-cresol. Lipopeptide 4 was purified using preparative RP-HPLC on the C4 Vydac column with a solvent gradient of 50-70% solvent B. Analytical high-performance liquid chromatography (HPLC)analysis was performed using Agilent instrument (C4 column): tR=27.6 min, purity >95%. ESI-MS: [M+2H]2+ m/z 2102.6 (calculated 2103.5); [M+3H]3+ m/z 1402.9 (calculated 1402.7); [M+4H]4+ m/z 1052.7 (calculated 1052.2); [M+5H]5+ m/z 842.2 (calculated 842.0); [M+6H]6+ 702.3 (calculated 701.8). MW=4205.1.
Lipopeptide 5
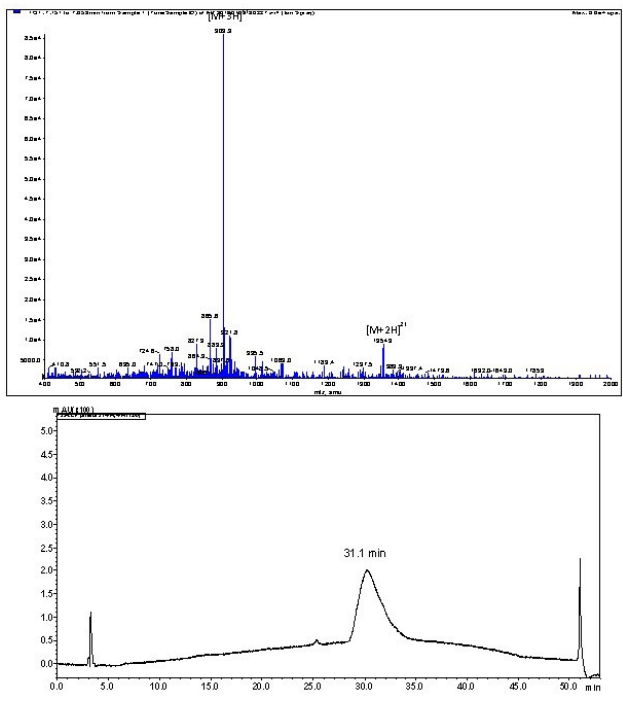
Lipopeptide 5 was synthesized using Fmoc-SPPS on a 0.2 mmoL scale (substitution ratio: 0.79 mmoL/g, 0.253 g) using rink amide MBHA resin.6 Following the same procedure as above, Dde-C16- OH lipoamino acids and remainder amino acids were coupled to the resin. The lipopeptide was purified using preparative RPHPLC on a C4 Vydac column with a gradient of 55-85% solvent B over 30 min. Analytical analysis was preformed using a Shimadzu instrument (C4 column): tR=31.1 min, purity >95%. Yield: 36%. ESI-MS: m/z 1355.2 (calculated 1354.5) [M+2H]2+; 903.5 (calculated 903.3) [M+3H]3+. MW=2707.4.
Formulation of Antigen-Loaded Nano-Liposomes
DPPC, DDAB, and cholesterol were dissolved in chloroform to final concentrations of 10 mg/mL, 5 mg/mL and 5 mg/mL, respectively, at a weight ratio of 5:2:1 prior to formulation.15 The antigen was dissolved at a concentration of 1 mg antigen in 1 mL of sterile MilliQ water. The dissolved lipids and antigen were combined, and the chloroform was slowly evaporated under reduced pressure using a rotatory evaporator. Any residual chloroform was removed under vacuum overnight. The liposomes were rehydrated using the lipid hydration method, with 1 mL of milliQ water at 56 °C following extrusion with 100 nm pore size polycarbonate membrane.
Characterization of Lipopeptides and Lipopeptide-Anchored Nanoliposomes
Liposome size was measured by dynamic light scattering (DLS) using a zetasizer 3000TM (Malvern Instruments, Malvern, UK).
Vaccination Scheme and Hookworm Challenge Model
Ten-week-old male in-bred BALB/c mice (Animal Resources Centre, Perth, WA, Australia) were randomly divided into groups of 10 and intranasally immunized on days 0, 16, and 41 with 10 μL (5 μL/nostril) of freshly prepared vaccine formulation. The formulation contained a mixture in a 1:1 ratio of B-cell epitope and T-helper epitope with a total of 10 μg antigen administered per mouse. Positive control mice were intranasally immunized with 10 μg of p3-K-P25 peptide mixed with 10 μg of cholera toxin subunit B (CTB) dissolved in 10 μL of water (5 μL/nostril). Negative control mice were immunized with 10 μL (5 μL/nostril) of phosphate-buffered saline (PBS). Prior to immunization, the mice were anaesthetized with isoflurane gas. The positive control CTB+p3-P25 and negative control PBS were administered to mice orally in parallel as described previously.6
Two-weeks after the final immunization (day 56), mice were infected with 500 infective Nippostrongylus brasiliensis larvae (L3) in 200 µl of PBS subcutaneously in the scruff. N. brasiliensis were maintained in Sprague–Dawley rats (Animal Resources Centre, Perth, WA, Australia), as previously described.18,19 Infective L3 were freshly prepared from 2-week-old rat fecal cultures. Sevendays post-infection, mice were euthanized with CO2. Blood, adult parasites from the small intestines, and weighed fecal samples from the large intestines were collected.
Collection of Serum
Blood samples were collected on days 18, 43 (pre-challenge) and 62 (post-challenge). Whole blood was collected into separation Z-Gel micro tubes containing clotting activator (Sarstedt) and centrifuged for 5 min at 10,062 x g and serum was collected. Pre-challenge serum samples were collected prior to challenge infection, and post-challenge blood samples were collected after euthanasia via cardiac puncture. All samples were stored at -80 °C prior to analysis.
Evaluation of Humoral Immune Response Against Specific Antigens
Enzyme-linked immunosorbent assays (ELISA) were performed to determine the antigen-specific IgG antibody titers in serum samples. All reactions were performed with 100 µL/well. Each well of a 96-well microtiter plate (Greiner Microlon® 600, Noida, India) was coated with 5 μg/mL of either p3 peptide or J14 peptide antigen (unrelated B-cell epitope from Group A Streptococcus bacteria; production has been reported elsewhere15) in 0.1 M sodium carbonate/bicarbonate (pH 9.6) for 90 min at 37 °C. The plates were washed three times with PBS-tween 20 (PBST; 0.05%) and blocked with 5% skim milk to reduce non-specific binding overnight at 4°C. Samples at a 1:100 dilution for IgG in serum were added for 90 min at 37 °C. The plates were washed three times and horseradish peroxidase conjugated goat anti-mouse secondary antibody (anti IgG at 1:4,000 in PBST, Invitrogen #62-6520) was added for 90 min at 37 °C. The plates were washed four times, then incubated with TMB substrate solution (Invitrogen 00-4201-56) at room temperature for 20 min in the dark. Antibody levels were considered significant when OD values exceeded an absorbance of the mean plus 3 standard deviations from the negative control mice.
Statistical analysis of the antibody titers between groups was performed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. Statistical analysis of worm and egg burden was performed using a non-parametric Mann-Whitney u test. GraphPad Prism 7.03 software (Graphpad Software Inc., CA, USA) was used for statistical analysis. Differences were significant if p<0.05.
Ethics Statement
The James Cook University (JCU) animal ethics committee approved all experimental work involving animals (ethics approval numbers A2300 and A2433). Animals were raised at the JCU animal facility under normal conditions of regulated temperature and lighting (12-hour light/dark cycle) with free access to pelleted food and water in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and the Queensland Animal Care and Protection Act.
RESULTS
Synthesis and Characterization of Nano-Liposomes and Lipopeptides
The p3 peptide was previously shown to stimulate mucosal and systemic immune responses.4,6 The J14 B-cell epitope, derived from bacterial group A Streptococcus (GAS), was used as an unrelated control to debar unspecific background response. This peptide was selected as a control, because its lipidated derivative was able to induce opsonic immune responses against GAS upon intranasal delivery in mice.20,21 The peptides and lipopeptides (Figure 1) were synthesized and purified. Lipopeptides 1, 2, 3, 4 and 5 were either formulated into cationic liposomes or allowed to selfassemble, and the particle size, zeta potential, and polydispersity of the nanoparticles were measured (Table 1). The liposome-based vaccine candidates (Lip1, Lip2, Lip3, Lip4) produced uniform, homogenously sized particles (PDI<0.02) with a size range of 150 nm to 170 nm (Table 1). In contrast, a mixture of 1+5 and 3+5 formed highly polydispersed particles in the solution.
| Table 1. Physicochemical Properties of the Vaccine Candidates, as Measured by Dynamic Light Scattering |
|
Formulation
|
Particle Size (nm) |
Zeta Potential (mV) |
PDI
|
|
Blank liposomes
|
155±<0.1 |
+51±1 |
0.07±0.02 |
| Lip1 |
148±2 |
+48±<1 |
0.06±0.02
|
|
Lip2
|
171±3 |
+48±<1 |
0.04±0.01 |
| Lip3 |
174±1 |
+49±<1 |
0.05±0.02
|
|
Lip4
|
167±1 |
+47±<1 |
0.04±0.01 |
| Lipopeptide 1+5 |
200±20
400±50 |
+35±2 |
0.44±0.1
|
|
Lipopeptide 3+5
|
100±60
520±230
5000±670 |
+37±2 |
0.66±0.1 |
Immunogenicity of Nanoliposomes and Lipopeptides
Mice immunized with control lipopeptides in liposomal formulation (Lip3 and Lip4) stimulated production of significant IgG levels against J14 peptide when administered intranasally (Figure 7A).22 Mice immunized with p3-bearing Lip2 and positive control cholera toxin B subunit (CTB)+p3-P25 produced low but significant levels of serum antibodies (Figure 7B-D). Mice vaccinated with p3-bearing vaccine candidates did not generate antibodies against J14 peptide, as expected. Immunized mice challenged with the N. brasiliensis parasite demonstrated no significant reductions in worm or egg burdens, with levels similar to the negative control group (Figure 8). Mice vaccinated with CTB+p3-P25 positive control also showed no protection against worms and had a similar egg burden to mice administered with PBS.
Figure 7. The Pre-challenge (day 18, day 43) and Post-challenge (day 62) IgG Antibody Levels
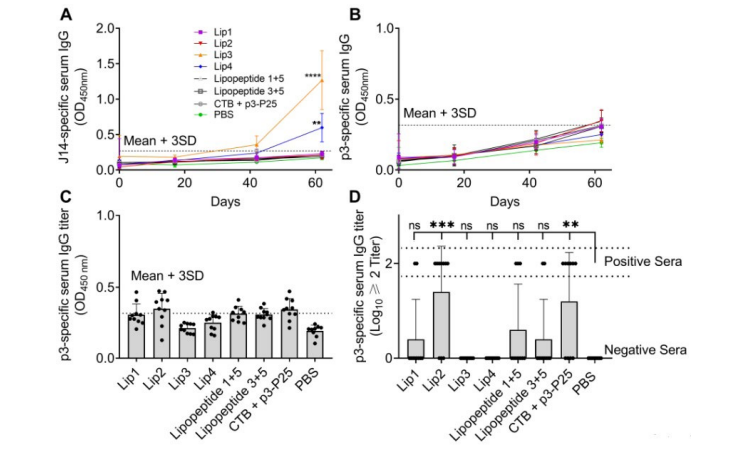
(A) J14-specific serum IgG (expressed as OD450) on days 18, 43 and 62. (B) p3-specific serum IgG (OD450) on days 18, 43 and 62; (C) p3-specific serum IgG (OD450) on day 62 (D) p3-specific serum IgG presented as Log10≥2 titer on day 62. Lip1, Lip2, Lipopeptide 1+5 and Lipopeptide 3+5 contain p3 peptide antigen, Lip3 and Lip4 contain J14 peptide antigen as unrelated controls. Dotted lines represent the mean+three standard deviations. Data represents mean±SD, (n=10 mice/group). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc multiple comparison test (**, p<0.01; ***, p<0.001). Statistical analysis was performed for mice immunized with peptide antigens in comparison to PBS-treated mice.
Figure 8. Parasite (adult worm and egg) Burden were Analyzed 7 Days Post-infection with Nippostrongylus Brasiliensi
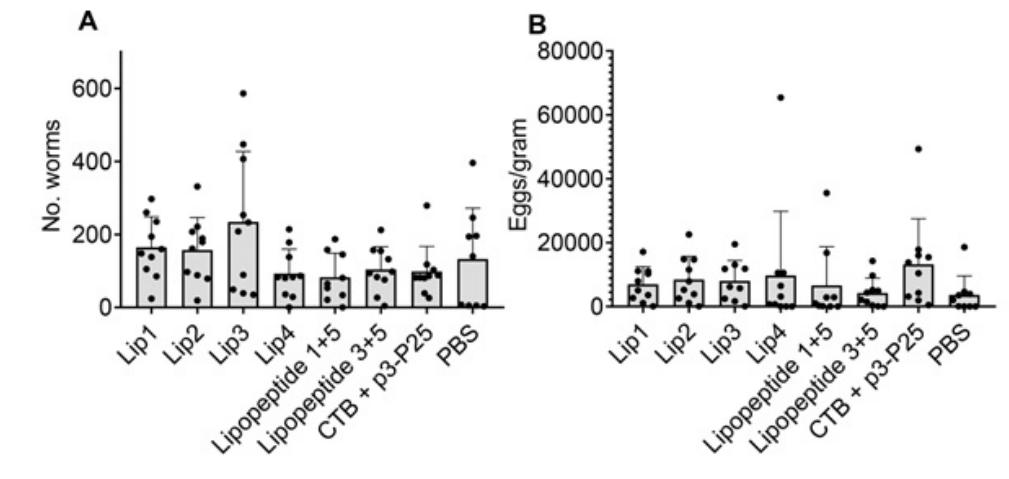
(a) The number of worms in the small intestines; (b) the number of eggs in fecal samples from the colon. The egg burden was calculated based on the number of eggs in a gram of feces (eggs/gramData represents mean±SD, (n=10 mice/group). Statistical analysis was performed using non-parametric Mann-Whitney u test. Statistical analysis was performed for mice immunized with peptide antigens in comparison to PBS-treated mice.
DISCUSSION
Bare peptides generate a nominal immune response when applied as a vaccine, so require a strong adjuvant for efficacy. The attachment of lipids to peptides in vaccine delivery systems has been shown to increase the immunogenicity of peptides for successful disease protection.23,24,25,26,27 Using two commonly studied lipids, Pam2Cys and LCP, we investigated the ability of lipopeptides to stimulate the production of protective antibodies against hookworm parasites after intranasal immunization in mice. As hookworms are complex parasites with lung-migratory larval and gastrointestinal adult stages, a mucosal (intranasal) vaccine may be ideal to stimulate both systemic and mucosal immunity. However, intranasal delivery of hookworm vaccine has had limited investigation.
The vaccine candidates in this study were designed to trigger production of anti-p3 antibodies, which were then expected to neutralize APR-1 enzyme and starve hookworm to death. The addition of a T-helper epitope is required to increase antibody production and memory response in outbred populations. B-cell and T-helper epitopes (here, p3 and P25 respectively) need to be delivered together to stimulate a strong immune response; however, instead of classical chemical conjugation, they can be co-delivered in liposomes or nanoparticles.15 The lipopeptides tested here were either anchored into liposomes or self-assembled into nanoparticles under aqueous conditions. This allowed for the formation of vaccine candidates as particles (Table 1). It has been suggested that smaller-sized particles are taken up via endocytosis into dendritic cells, while larger particles are phagocytosed by the mononuclear phagocyte system.28,29 Charge is also an important factor in liposome formulation and immune response stimulation. Positively charged liposomes can act as a depot, retaining antigen at the injection site for longer, causing a sustained release of antigen to antigen presenting cells (APCs), while smaller particles travel directly to the lymph nodes for processing.30 Positively charged liposomes can also adhere to the negative charge present on the membranes of APCs, enhancing endocytosis.20,31 The liposomal formulations employed in this study had size (<200 nm) and charge(<+60 mV), which was previously shown to be safe and effective in vaccine delivery against group A Streptococcus, human papilloma virus, and malaria.15,32,33 Lipopeptides were also selfassembled in aqueous solution, without the liposome delivery system, and formed particles with broad size distribution (Table 1). To investigate the efficacy of the vaccine candidates, a mouse model for hookworm infection was used. Mice are not ideal hosts for the human hookworm Necator americanus. However, transcriptome and secreted proteome studies into N. americanus demonstrated significant sequence homology with a nematode parasite, Nippostrongylus brasiliensis, commonly known as “rodent hookworm”.34,35 The sequence of p3 epitope is identical (100% conserved) between rodent hookworm (Nippostrongylus brasiliensis) and human hookworms (Necator americanus, Ancylostoma duodenale, and Ancylostoma ceylanicum).36 Thus, N. brasiliensis was used as the challenge model in this study. Only some individual mice immunized with p3-specific vaccine candidates stimulated significant levels of serum antibodies against the p3 peptide compared to the PBS group. Mice vaccinated intranasally with similar concentrations of J14 B-cell epitope, unrelated to hookworm, produced IgG against the J14 peptide at levels similar to those described previously, protective against the pharyngitis inducing group A beta-hemolytic streptococcus (GAS).22 By a different route of administration, mice orally immunized with the positive control and Lip2 vaccine candidates have been shown to generate a humoral immune response against both peptide antigen, and the parent protein in the hookworm gut and resulted in significant decrease in mean intestinal worm and egg burdens.6 Here, intranasally immunized mice were not able to produce significant antibody titers against p3 peptide and no protection against N. brasiliensis parasite challenge was observed. Therefore, the low-level of p3-specific antibodies was unable to influence worm and egg burden in vivo. Wisniewski et al37 demonstrated that intranasal immunization of hamsters using a cDNA vaccine encoding a protein derived from metalloprotease 6 from another hookworm species, Ancylostoma ceylanicum, reduced worm burden by 80% after single-dose intranasal administration, whereas triple immunization failed to significantly reduce worm burden. The mechanism of lower efficacy is unknown; however, it was suggested that the vaccine construct may have induced immune tolerance or immunosuppression. Further studies from Wisniewski et al37 demonstrated that three immunizations with A. ceylanicum metalloprotease 7 cDNA vaccine via the nasal route significantly reduced worm and egg burden in hamsters.38 However, the immunization dose (50 μg/immunization), volume and animals (hamsters)37,38 used in that study differ from presented here. Further, promising vaccine candidates administered to different sites may not elicit the desired immune responses.39,40 Therefore, to overcome lack of desired efficacy of reported here mucosal hookworm vaccine several strategies can be examined: a) the current formulation can be modified, for example by incorporation of mucosa adhesive trimethyl chitosan41,42,43,44; b) the dose and number of immunization can be increased; c) hookworm species, stage of lifecycle, and animal model can be altered; or d) as the chosen peptide epitope p3 targets the protein responsible for degradation of host hemoglobin once parasite is inside the gut of the host, an oral administration route might be favourable to elicit desired mucosal and systemic immune response.45,46
CONCLUSION
In this study, lipopeptides anchored into liposomes or self-assembled into nanoparticles were examined for their ability to protect mice against hookworm infection after nasal immunization. The vaccine candidates were all produced to have optimal charge and particle size for immune stimulation. However, neither LCP nor Pam2Cys attached to p3 peptide epitope were able to stimulate significant serum antibody titers that protected mice against parasite infection. Interestingly, liposomes anchoring lipidated antigen were affective as delivery system for antibacterial vaccine, but not for hookworm vaccine, following intranasal administration. Moreover, the same antiparasitic lipopeptide-based vaccines when delivered orally was effective, but ineffective following intranasal immunization.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.