INTRODUCTION
Intestinal parasites are an important cause of morbidity and mortality although they usually create non-aggressive diseases and constitute a major public health problem in their transmission from person to person, especially in developing countries where poor sanitary conditions and lack of information result in the contamination of food and water sources with a consequent continuance of parasite cycles.1,2 Even in countries where adequate sanitation conditions and education excel, some of these parasites play an important role in causing diseases in specific groups such as immunocompromised individuals and young children.3 About 340 parasites infect more than three billion people worldwide with varying morbidity and mortality.4 They affect an estimated 3.5 billion persons and cause clinical signs and symptoms in approximately 450 million.5 The two main types of intestinal parasites are helminths and protozoa.
Diabetes mellitus (DM) is a metabolic non-communicable disease in which a person has high blood glucose, either because the body does not produce enough insulin or because cells do not respond to the insulin that is produced.6,7 There are two types of diabetes mellitus: Type 1, Insulin-Dependent Diabetes Mellitus (IDDM); Type 2, Non-Insulin-Dependent Diabetes Mellitus (NIDDM).8 This high blood sugar produces the classical symptoms of polyuria, polydipsia and polyphagia. Diabetes is one of the most frequent metabolic diseases and is widely distributed in various populations.8 Sub-Saharan Africa faces the world’s highest increase in type 2 Diabetes occasioned by adaptation to western lifestyles and genetic pre-dispositions.9 Diabetic patients have been reported to be immunocompromised.1,10,11,12 Intestinal parasites have gained increasing attention as important opportunistic pathogens responsible for clinically important infections in immunosuppressed patients.13,14
The paucity of information on the prevalence and type of pathogenic intestinal parasitic infection in diabetic patients in Cameroon prompted this study and will provide useful up-to-date data for parasitic infection in diabetic patients.
MATERIALS AND METHODS
Study Area
The study was conducted in Buea and Limbe, two important cities in the Fako division and South West (SW) region of Cameroon. Buea is located about 800 m above sea level in Mount Cameroon. Buea, the capital of the SW region is located on the eastern slopes of Mount Cameroon and has a population of over 200,000. It lies at latitudes 4.09 °N and longitudes 9.13 °E and has a total surface area of 870 km2. Limbe formally known as Victoria is a natural resource coastal city situated at 4.00 °N and 9.11 °E. It covers a surface area of 549 km2 and situated near the Atlantic Ocean. Limbe has an equatorial climate and is dominated by the tropical equatorial rainforest with tall trees.
Study Population
The study focused on diabetic patients who live and visit diabetic units in hospitals and clinics in the study area. The diabetic patients were controlled patients who visited the diabetic unit for routine checkup. Non-diabetic individuals in the study area served as a control group.
Study Design
A hospital based cross-sectional study was carried out from March to June 2015 to determine the prevalence of intestinal parasites in diabetic patients. Non-diabetics served as a control group.
Ethical Considerations
The ethical approval of the study was sought and obtained from the Ethical Review committee of the Faculty of Health Science Institutional Review Board (FHS-IRB) of the University of Buea. Administrative clearance was also sought and obtained from the South West regional delegation for public health and from the director of the Buea Regional Hospital annex and the Limbe Regional hospital after presentation of detailed study objectives and procedures. Written informed consent was gotten from each recruited study participant from 21 years and above with participation being voluntary.
Data Management and Analysis
During data collection completed questionnaires were checked regularly to rectify any discrepancy, logical errors or missing values. Participants data obtained were entered into a log book, and later keyed into a computer using Microsoft excel 2013 and verified for the possibility of entering errors. Data were coded, entered and analyzed using Statistical Package for Social Sciences (SPSS) version 20. Continuous variables were described using mean and standard deviation, and categorical variables using their frequency and percentage. Chi-square test was used to test level of significance at p-value <0.05 considered statistically significant.
Data Collection
After obtaining consents from the participants a structured questionnaire was administered by trained personnel to collect clinical information and socio-demographic characteristics.
Specimen Collection and Laboratory Procedures
Stool specimen was collected from each participant in a dry, clean, leak proof, tight lid plastic container containing a small spoon labelled with an identification number.
Stool samples were analyzed by direct microscopy followed by the formol-ether concentration technique for microscopical detection of intestinal parasites. The modified ziehlnelsen method was used to detect intestinal coccidians Cryptosoporidiumparvum, Isospora belli and Cyclosporacayetanensis. Stoll’s technique was used for the quantification of helminth eggs.13
A drop of blood was gotten from non-diabetic participants using a lancet and analyzed using a glucometer for random blood sugar to confirm they are actually not diabetic. They were also asked if they experienced any of the signs and symptoms of diabetes mellitus. Non-diabetic participants with a random blood sugar of 200 mg/dl or greater and had common signs and symptoms of diabetes was considered diabetic and referred to a diabetologists.
RESULTS
A total of 235 participants were recruited. 150 diabetic patients and 85 non-diabetic individuals (control group) with data on socio-demographic characteristics collected from diabetic patients only.
Among the 150 diabetic participants, 105(70%) were females while 45(30%) were males. Their age ranged from 27- 90 years with a mean age of 56.1 years (Standard Deviation (SD)=11.8)). Most of the participants were between the age group of 41-60 years. The mean age of participants in the control group was 29.28 years (SD=7.7) and their ages ranged from 21-63 years. There were 35(41.2%) females and 50(58.2%) males.
Intestinal parasites were diagnosed in 15 out of 150(10%) participants in the diabetic group with some having mixed or co-infections. Figure 1 shows that five different intestinal parasites were identified from the study participants with three protozoans (E. histolytica, B. hominis and Cryptosporidium parvum) and two helminthes (Ascaris and hookworm) identified. Figure 1 also show that E. histolytica 10(6.7%) was the pre-dominant parasite identified from stool of the study participants followed by B. hominis 4(2.7%). One (0.67%) each of A. lumbricoides, Hookworm and Cryptosporidium parvum was also identified from study participants. Females showed a higher prevalence with parasitic infections 11(10.5%) than males 4(8.9%). The prevalence of parasitic infections was higher from the urban areas 10(11.0%) than from the rural areas 5(8.5%). The age group 41-60 years showed the highest prevalence 11(13.3%).
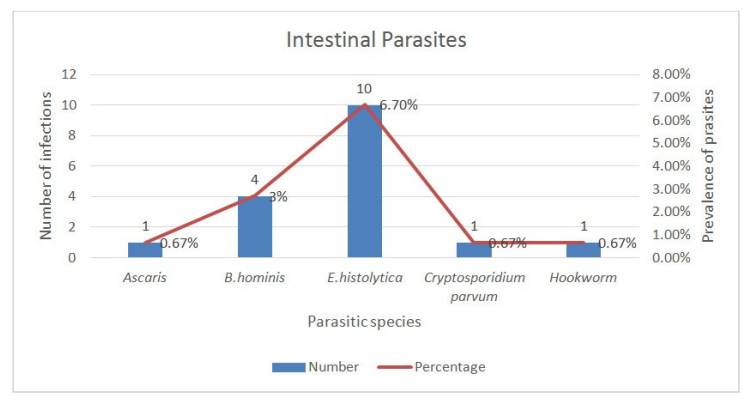
Figure 1: Intestinal parasites in diabetes mellitus patients.
In the control group, 20 stool samples were positive for intestinal parasites 10(20%) males, 10(28.6%) females, with the following prevalence: E. histolytica 18(21.2%), A. lumbricoides 2(2.4%), and B. hominis 1(1.2%) as shown in Figure 2. An overall prevalence of 23.5% of intestinal parasites was observed in the control group. Polyparasitism was observed in only one control patient. A. lumbricoides was found only in the male sex.
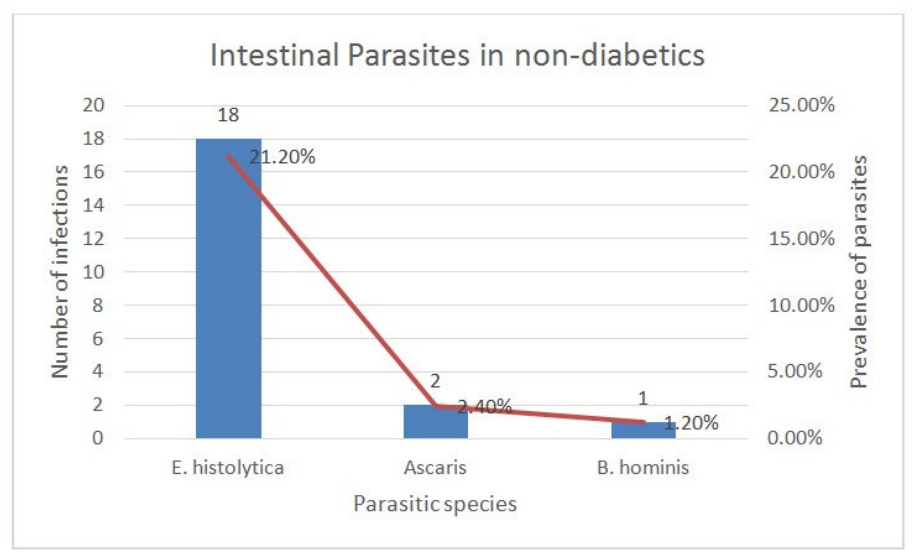
Figure 2: Intestinal parasites in non-diabetic patient.
Intestinal Protozoa
Three different intestinal protozoans were identified from the diabetic study participants. 13(8.6%) of diabetes mellitus participants were infected with intestinal protozoans with E. histolytica being the most prevalent 10(6.7%) followed by B. hominis 4(2.7%) while the intestinal coccidian Cryptosporidium parvum 1(0.7%) was the least prevalent. Two different intestinal protozoans were identified from the non-diabetic individuals which are E. histolytica 18(21.2%) and B. hominis 1(1.2%).
9 females (8.5%) were infected with intestinal protozoan and 4 males (8.9%). The prevalence of intestinal protozoans was higher in males than in females. E. histolytica was predominant amongst the females and had the only coccidian parasite C. parvum.
In the control group, 10 females and 9 males were infected with intestinal protozoa with E. histolytica being the predominant intestinal protozoa found in more females than males.
Male gender had the only B. hominis identified.
Participants from the Urban areas 9(9.9%) were more infected with intestinal protozoans than those from the rural areas 4(6.7%). E. histolytica is the predominant intestinal protozoan which was more prevalent in the urban areas than rural areas. B. hominis mono-parasitism was observed only in rural areas while a co-infection of E. histolytica and B. hominis was identified only in urban areas.
Peak values of intestinal protozoa were obtained in age group 41-60 years. All three intestinal protozoans were identified in the age group 41-60 years with E. histolytica dominating and only E. histolytica was identified in the age group 61-80 years.
Intestinal Helminths
A total of two different intestinal helminthes was identified among diabetic participants with a prevalence of 2(1.3%). The helminthes were A. lumbricoides and hookworm and they were one each amongst diabetic patients with a prevalence of 0.7% per helminth.
Only one intestinal helminth was identified among non-diabetic individuals and that was Ascarislumbricoides with a prevalence of 2(2.4%).
The infection intensity of helminthes eggs was light infections. Classifications were done as specified by the World Health Organization (WHO) classification scheme shown in Table 1. Both Ascaris and hookworm had light infection intensities. Ascarislumbricoides had an egg count of 300 eggs per gram of faeces while hookworm had an egg count of 100 eggs per gram of faeces. Ascaris had a higher egg count than hookworm.
| Table 1: WHO classification scheme for intestinal helminthes intensities. |
|
Helminthes Species
|
Light(Epg) |
Moderate(Epg) |
Heavy(Epg)
|
|
Ascarislumbricoides
|
1-4,999 |
5000-49,999 |
>50,000 |
|
Hookworm
|
1-1999 |
2000-3,999 |
≥4,000
|
The Ascaris in the control group had an egg count of 100 eggs per gram of faeces in both non-diabetic individuals hence light infection intensities.
Single and Mixed Parasitic Infections in Diabetic Patients
Multi-parasitism existed only among the protozoa species with two pathogenic protozoa co-infection and a pathogenic and non-pathogenic protozoa co-infection. Helminthes had only single infection or mono-parasitism. Table 2 shows that single infections 9(6%) were more prevalent than mixed infections 6(4%). E. histolytica recorded the highest single and multiple infections. Non-pathogenic E. coli existed only as co-infections with other parasites or protozoa. Furthermore, there were no cases of helminthes/helminthes co-infection.
| Table 2: Frequency of single and multiple infections. |
|
Parasites
|
Number of patients with parasites (%)
|
|
Single Infections
|
B. hominis
|
2(1.3) |
| E. histolytica |
4(2.7)
|
|
C. parvum
|
1(0.7) |
| A. lumbricoides |
1(0.7)
|
|
Hookworm
|
1(0.7)
|
|
Total
|
|
9(6)
|
|
Multiple Infections
|
B. hominis+E. histolytica+E.coli
|
1(0.7) |
| E. histolytica+E. coli |
3(2)
|
|
B. hominis+E. histolytica
|
1(0.7) |
| E. histolytica+E. coli+Trichomonashominis |
1(0.7)
|
|
Total
|
|
6(4)
|
Prevalence of Intestinal Parasitic Infections in Diabetes Mellitus Patients vs. Non-Diabetes Mellitus Individuals
Comparing the prevalence of intestinal parasites in the diabetic group versus prevalence in the non-diabetic group (10% vs. 23.5%) it was seen that the prevalence was statistically significant with a p-value less than 0.05.
Association and Relationship between DM Status and Intestinal Parasitism
Table 3 shows that the association between DM status and infection with parasites is statistically very significant. A protective association exist between Diabetes mellitus and Intestinal parasitic infections.
Diabetes protects against getting intestinal parasitic infections. Table 3 shows an odd ratio of 0.36 which indicates a decreased risk for diabetes mellitus patients to acquire intestinal parasitic infections.
| Table 3: Relationship between DM status and Intestinal parasitic infections. |
|
Diabetes mellitus status DM patients
|
Number tested 150 |
Number infected (%) 15(10) |
Odd ratio 0.36 |
95% CI 0.17-0.75 |
P-value 0.0051
|
| Non-DM individuals |
85 |
20(23.5) |
|
|
|
DISCUSSION
The prevalence rates of intestinal parasites, including opportunistic protozoa, in Africa vary from study to study depending on the diagnostic technique used and the study population.15 An overall prevalence of 10% of intestinal parasitic infection was observed among diabetic patients which is lower than the prevalence reported in the south western part of Nigeria (18.7%)8 and South east Turkey (47%).2 Geographical location may account for this difference as the Olusegun et al8 study was carried out in Nigeria and the Nazligul et al2 study was carried out in Sanliurfa province which was an endemic zone for intestinal parasites.
Five different intestinal parasites were identified from the study participants with three protozoan (E. histolytica, B. hominis and Cryptosporidium parvum) and two helminthes (Ascaris and hookworm) identified. This differs from the study carried out in Nigeria8 where three different intestinal parasites were identified and Hookworm being the most prevalent while E. histolytica is the least prevalent. It also differs from the study carried out in South east Anatolia2 or Turkey where same number of different parasites where identified but the only protozoa were E. histolytica, Giardia lamblia and helminthes were Ascaris, Trichuristrichuria and Taenias. Ascaris was the most prevalent intestinal parasite. Helminthes were the least prevalent intestinal parasite in this study due to the massive drug administration of anti-helminthics by the government of Cameroon recently to help eradicate intestinal helminthes in the nation but intestinal protozoa still pose a threat.
This investigation reveals that there are more female participants who were also more infected (10.5%) with intestinal parasites than males (8.9%). This can be explained by the fact that there is a high prevalence of diabetes mellitus occurring in females than in males and this is similar to the study in Nigeria11 and Sanliurfa province.2 Also females are more engaged in farming and domestic work which exposes them to these intestinal parasites. Also there were parasitic infections in the age groups 41-60 years (13.3%) and 61-80 years (7.8%). In the other age groups there were no parasitic infections discovered. This could be due to the weakened immune system that comes with ageing coupled with diabetes mellitus status. Results were similar to those obtained in Nigeria8 where high prevalence was obtained in the age group 51-60 years followed by 41-50 years and 61-70 years.
There were more parasitic infections in the urban areas (10%) than rural areas (8.5%) probably due to the migration from the rural to urban areas of diabetic patients to meet their children so that they can be well taken care of and taken to the hospital regularly for checkup. E. histolytica was predominant in the urban areas probably through fruit and food (vegetables) handlers who sell fruits and food by the road side in the urban towns than in the rural areas. This food and fruits are mostly bought by diabetic patients especially after fasting due to blood sugar control from the nearest vendor and they are also advised to eat vegetables. This same study shows a high prevalence of E. histolytica in the general non-diabetic population which serves as a reservoir host for transmission of E. histolytica. Intestinal parasites were more prevalent among business people and civil servants probably due to their busy schedules which makes it difficult for them to visit the doctor regularly. Also they spend most of their time out of their homes hence possibly feeding out of the house from places where sanitary conditions are questionable.
The prevalence of intestinal parasites in the control group or non-diabetic individuals (23.5%) was higher in this study and statistically different or lower than the prevalence in the diabetic group (10% vs. 23.5%, p=0.0052). This was similar with the findings of Nazligul et al2 who found out that intestinal parasite prevalence in the diabetic group was found to be significantly lower than in the control subject group (47 vs. 55%, p<0.05). This can be explained by the greater number of physician visits incurred by diabetic patients than the non-diabetic patients where diabetic patients consult frequently and are treated for possible intestinal parasitic infections. This differs from the study by Olusegun et al8 where non-diabetics had no intestinal parasites probably due to the low prevalence of intestinal parasites in that region. The presence of Cryptosporidium only in the diabetics reinforces the theory of diabetics being immunologically weaker than non-diabetics.
DM status was significantly associated with the prevalence of intestinal parasites or acquiring intestinal parasitic infection (OR: 0.36 CI=0.17-0.75; p=0.0051). Being diabetic was not a risk factor for acquiring intestinal parasitic infection but instead decreases your chances of acquiring the parasites due to a higher prevalence of intestinal parasites in the control group. This differs from the findings of Olusegun et al13 where being diabetic was a risk factor for acquiring intestinal parasites. This is due to the fact that the standard of living in this communities are better, sanitation is better, diabetic patients visit the doctor regularly, consult, follow the doctor’s order diligently and are well taken care of by their family members since most diabetic patients are old.
CONCLUSION
The prevalence of IPIs (Intestinal Parasitic Infections) among Diabetes mellitus patients in Limbe and Buea communities obtained was 10.0%. The parasites detected in diabetics included Entamoebahistolytica, Blastocystishominis, Ascarislumbricoides, Hookworm and Cryptosporidium parvum. Three protozoans and two helminthes. The most prevalent type of intestinal parasite in diabetics is Entamoebahistolytica which is same with non-diabetics. The more types of intestinal parasites in diabetics and along with the detection of C. parvum indicates a weakened immune system in diabetics. Our study suggests a protective association exist between Diabetes mellitus and Intestinal parasitic infections. Diabetic patients should be screened routinely for intestinal parasites especially protozoans and treated for their overall wellbeing.
ACKNOWLEDGEMENTS
The author profound gratitude goes to his parents Mr. Fominyam Christopher and Mrs. Fominyam Margaret for their financial and moral support for the success of this work. Thanks to all the diabetes patients attending the Buea and Limbe Regional Hospital Diabetic unit who accepted to participate in this study. Author reserves special thanks to Mr. Meli Rigobert, Mr. Nkwenty, Mr. Ejem and Miss Mirabel for their technical assistance and genuine criticism throughout this work. Thanks to Dr. Fogwe Boniface, the Director of Viktoria Hope Foundation for his guidance and approval to work in his laboratory. Special thanks to the Director of Limbe, Dr. Kuwoh Pius and Buea Regional Hospitals and the HOD of Medical Laboratory Sciences, University of Buea Dr. Assob Nguedia for their approvals to work in their hospitals and laboratories.
AUTHORS’ CONTRIBUTIONS
FBT, NAL and EBF planned the study and designed the protocols. NAL and EBF supervised the study including collection of stool samples, data from the questionnaire interviews and management of collected data. FBT supervised the laboratory work and administered questionnaires to participants. FBT and EJE carried out the data analysis and interpretation. FBT prepared the first draft of the manuscript and all the authors revised the manuscript critically.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







