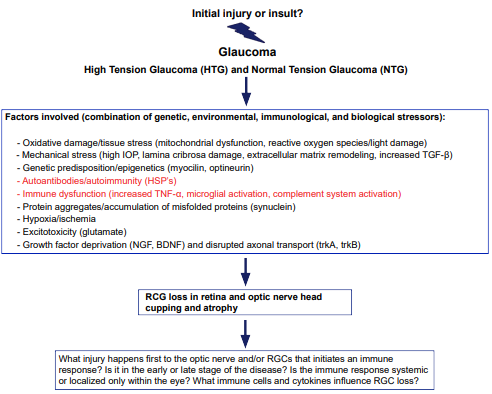
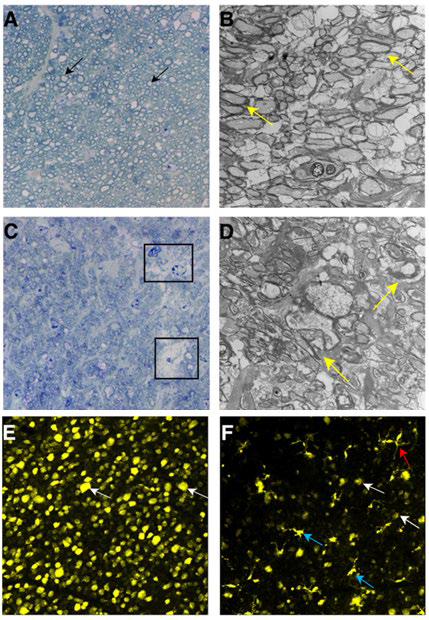
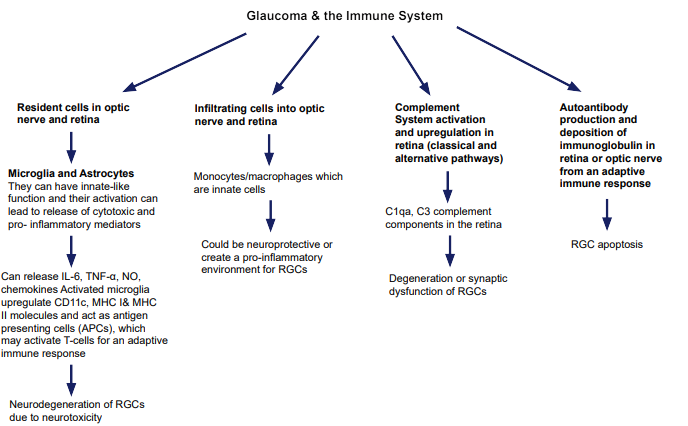
1. Wax MB. Is there a role for the immune system in glaucomatous optic neuropathy? Curr Opin Ophthalmol. 2000; 11(2): 145-150. doi: 10.1097/00055735-200004000-00014
2. Russo R, Varano GP, Adornetto A, et al. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur J Pharmacol. 2016; 787: 134-142. doi: 10.1016/j. ejphar.2016.03.064
3. Tezel G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp Eye Res. 2011; 93(2): 178-186. doi: 10.1016/j.exer.2010.07.009
4. Bell K, Gramlich OW, Von Thun Und Hohenstein-Blaul N, et al. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog Retin Eye Res. 2013; 36: 199-216. doi: 10.1016/j. preteyeres.2013.02.003
5. Fan W, Li X, Wang W, Mo JS, Kaplan H, Cooper NG. Early involvement of immune/inflammatory response genes in retinal degeneration in DBA/2J mice. Ophthalmol Eye Dis. 2010; 2: 23-41. doi: 10.4137/oed.s2854
6. Gramlich OW, Bell K, von Thun Und Hohenstein-Blaul N, et al. Autoimmune biomarkers in glaucoma patients. Curr Opin Pharmacol. 2013; 13(1): 90-97. doi: 10.1016/j.coph.2012.09.005
7. Grus F, Sun D. Immunological mechanisms in glaucoma. Semin Immunopathol. 2008; 30(2): 121-126. doi: 10.1007/s00281- 008-0105-8
8. Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007; 184(1-2): 100- 112. doi: 10.1016/j.jneuroim.2006.11.019
9. Huang P, Zhang SS, Zhang C. The two sides of cytokine signaling and glaucomatous optic neuropathy. J Ocul Biol Dis Infor. 2009; 2(2): 78-83. doi: 10.1007/s12177-009-9034-6
10. Joachim SC, Grus FH, Kraft D, et al. Complex antibody profile changes in an experimental autoimmune glaucoma animal model. Invest Ophthalmol Vis Sci. 2009; 50(10): 4734-4742. doi: 10.1167/iovs.08-3144
11. Kipnis J, Schwartz M. Controlled autoimmunity in CNS maintenance and repair: Naturally occurring CD4+CD25+ regulatory T-cells at the crossroads of health and disease. Neuromolecular Med. 2005; 7(3): 197-206. doi: 10.1385/NMM:7:3:197
12. Knier B, Rothhammer V, Heink S, et al. Neutralizing IL-17 protects the optic nerve from autoimmune pathology and prevents retinal nerve fiber layer atrophy during experimental autoimmune encephalomyelitis. J Autoimmun. 2015; 56: 34-44. doi: 10.1016/j.jaut.2014.09.003
13. Moalem G, Gdalyahu A, Shani Y, et al. Production of neurotrophins by activated T cells: Implications for neuroprotective autoimmunity. J Autoimmun. 2000; 15(3): 331-345. doi: 10.1006/jaut.2000.0441
14. Rieck J. The pathogenesis of glaucoma in the interplay with the immune system. Invest Ophthalmol Vis Sci. 2013, 54(3): 2393-2409. doi: 10.1167/iovs.12-9781
15. Tezel G, Yang X, Luo C, et al. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 2010, 51(10): 5071-5082. doi: 10.1167/ iovs.10-5289
16. Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: Oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2007; 48(2): 705- 714. doi: 10.1167/iovs.06-0810
17. Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009; 88(4): 825-830. doi: 10.1016/j.exer.2009.02.005
18. Wax MB, Tezel G, Yang J, et al. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008; 28(46): 12085-12096. doi: 10.1523/JNEUROSCI.3200-08.2008
19. Ramirez AI, de Hoz R, Salobrar-Garcia E, et al. The role of microglia in retinal neurodegeneration: Alzheimer’s disease, parkinson, and glaucoma. Front Aging Neurosci. 2017; 9: 214. doi: 10.3389/fnagi.2017.00214
20. Williams PA, Marsh-Armstrong N, Howell GR, et al. Neuroinflammation in glaucoma: A new opportunity. Exp Eye Res. 2017; 157: 20-27. doi: 10.1016/j.exer.2017.02.014
21. Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: Naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002; 99(24): 15620-15625. doi: 10.1073/pnas.232565399
22. London A, Itskovich E, Benhar I, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011; 208(1): 23-39. doi: 10.1084/jem.20101202
23. Schwartz M. Neurodegeneration and neuroprotection in glaucoma: Development of a therapeutic neuroprotective vaccine: The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2003; 44(4): 1407-1411. doi: 10.1167/iovs.02-0594
24. Schwartz M. Modulating the immune system: A vaccine for glaucoma? Can J Ophthalmol. 2007; 42(3): 439-441. doi: 10.3129/can.j.ophthalmol.i07-050
25. Schwartz M, Shaked I, Fisher J, Mizrahi T, Schori H. Protective autoimmunity against the enemy within: Fighting glutamate toxicity. Trends Neurosci. 2003; 26(6): 297-302. doi: 10.1016/ S0166-2236(03)00126-7
26. Schwartz M. Physiological approaches to neuroprotection. boosting of protective autoimmunity. Surv Ophthalmol. 2001; 45(Suppl 3): S256-S260. doi: 10.1016/S0039-6257(01)00208-9
27. Schwartz M. Sell Memorial Lecture. Helping the body to cure itself: Immune modulation by therapeutic vaccination for spinal cord injury. J Spinal Cord Med. 2003; 26(Suppl 1): S6- S10. doi: 10.1080/10790268.2003.11753719
28. Schwartz M, Baruch K. Breaking peripheral immune tolerance to CNS antigens in neurodegenerative diseases: Boosting autoimmunity to fight-off chronic neuroinflammation. J Autoimmun. 2014; 54: 8-14. doi: 10.1016/j.jaut.2014.08.002
29. Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010; 6(7): 405- 410. doi: 10.1038/nrneurol.2010.71
30. Tamm ER, Ethier CR, Lasker IIoA; Glaucomatous Neurodegeneration Participants. Biological aspects of axonal damage in glaucoma: A brief review. Exp Eye Res. 2017; 157: 5-12. doi: 10.1016/j.exer.2017.02.006
31. Johnson TV, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010; 81(2-3): 349-358. doi: 10.1016/j.brainresbull.2009.04.004
32. Struebing FL, Geisert EE. What animal models can tell us about glaucoma. Prog Mol Biol Transl Sci. 2015; 134: 365-380. doi: 10.1016/bs.pmbts.2015.06.003
33. Bakalash S, Kipnis J, Yoles E, Schwartz M. Resistance of retinal ganglion cells to an increase in intraocular pressure is immune-dependent. Invest Ophthalmol Vis Sci. 2002; 43(8): 2648-2653.
34. Tezel G, Fourth APORICWG. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009; 50(3): 1001-1012. doi: 10.1167/iovs.08-2717
35. Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2015; 51: 1-40. doi: 10.1016/j.preteyeres.2015.06.003
36. Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006; 1088: 219-229. doi: 10.1196/annals.1366.012
37. Vidal-Sanz M, Nadal-Nicolas FM, Valiente-Soriano FJ, Agudo-Barriuso M, Villegas-Perez MP. Identifying specific RGC types may shed light on their idiosyncratic responses to neuroprotection. Neural Regen Res. 2015; 10(8): 1228-1230. doi: 10.4103/1673-5374.162751
38. Romano C, Barrett DA, Li Z, Pestronk A, Wax MB. Antirhodopsin antibodies in sera from patients with normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 1995; 36(10): 1968-1975.
39. Romano C, Li Z, Arendt A, Hargrave PA, Wax MB. Epitope mapping of anti-rhodopsin antibodies from patients with normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999, 40(6):1275-1280.
40. Tezel G, Edward DP, Wax MB. Serum autoantibodies to optic nerve head glycosaminoglycans in patients with glaucoma. Arch Ophthalmol. 1999; 117(7): 917-924. doi: 10.1001/ archopht.117.7.917
41. Tezel G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol. 2013, 13(1): 23-31. doi: 10.1016/j.coph.2012.09.013
42. Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998; 39(12): 2277-2287.
43. Wax MB. The case for autoimmunity in glaucoma. Exp Eye Res. 2011; 93(2): 187-190. doi: 10.1016/j.exer.2010.08.016
44. Kountouras J, Mylopoulos N, Boura P, et al. Relationship between Helicobacter pylori infection and glaucoma. Ophthalmology. 2001;108(3): 599-604. doi: 10.1016/S0161- 6420(00)00598-4
45. Astafurov K, Elhawy E, Ren L, et al. Oral microbiome link to neurodegeneration in glaucoma. PLoS One. 2014; 9(9): e104416. doi: 10.1371/journal.pone.0104416
46. Nikolskaya T, Nikolsky Y, Serebryiskaya T, et al. Network analysis of human glaucomatous optic nerve head astrocytes. BMC Med Genomics. 2009; 2: 24. doi: 10.1186/1755-8794-2-24
47. Stasi K, Nagel D, Yang X, et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006; 47(3): 1024-1029. doi: 10.1167/iovs.05-0830
48. Howell GR, Libby RT, John SW. Mouse genetic models: An ideal system for understanding glaucomatous neurodegeneration and neuroprotection. Prog Brain Res. 2008; 173: 303-321. doi: 10.1016/S0079-6123(08)01122-9
49. Howell GR, Macalinao DG, Sousa GL, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011; 121(4): 1429-1444. doi: 10.1172/JCI44646
50. Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001; 131(4): 421-426. doi: 10.1016/S0002- 9394(00)00862-X
51. Golubnitschaja O, Flammer J. What are the biomarkers for glaucoma? Surv Ophthalmol. 2007; 52(Suppl 2): S155-S161. doi: 10.1016/j.survophthal.2007.08.011
52. Gramlich OW, Beck S, von Thun Und Hohenstein-Blaul N, et al. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS One. 2013; 8(2): e57557. doi: 10.1371/journal.pone.0057557
53. Hammam T, Montgomery D, Morris D, Imrie F. Prevalence of serum autoantibodies and paraproteins in patients with glaucoma. Eye (Lond). 2008; 22(3): 349-353. doi: 10.1038/sj.eye.6702613
54. Cui Q, Yin Y, Benowitz LI. The role of macrophages in optic nerve regeneration. Neuroscience. 2009; 158(3): 1039-1048. doi: 10.1016/j.neuroscience.2008.07.036
55. Jung S, Schwartz M. Non-identical twins – microglia and monocyte-derived macrophages in acute injury and autoimmune inflammation. Front Immunol. 2012, 3: 89. doi: 10.3389/ fimmu.2012.00089
56. Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014; 2(1): 1. doi: 10.1186/2050-7771-2-1