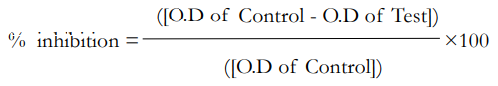INTRODUCTION
Since ancient times, plants have been an exemplary source of medicine. Ayurveda and other Indian literature mentioned the use of plants in treatment of various human ailments. According to Namita et al1 traditional system of medicine has been found to have many utilities in treatment of various diseases. The need for the use of plants to treat human disease could be attributed to several factors including, inadequate supply of drugs and high cost of treatment associated with conventional medical practices. Side effects coupled with drug resistance encountered with synthetic drugs also necessitated the need for alternative therapy. The affordability of herbals has also drawn the attraction towards their use. Moringa oleifera (Moringa (MO)) and Camelia Sinensis (green tea (GT)) are two of such medicinal plants gaining popularities for their reported claims on health benefits in recent years.2
Moringa oleifera is one of the most widely distributed species of the Moringaceae family throughout the World, especially in Asian countries, having a remarkable range of pharmacological properties in addition to significant nutritional value. Moringa oleifera is a highly valued plant in tropical and subtropical countries where it is mostly cultivated.2 The medicinal properties of the plant’s edible parts have been recognized by both the Ayurvedic and Unani systems of medicine in India.3
Camelia Sinensis (Green tea) has been associated with lowering the risk of cancer, lowering the risk of coronary heart disease and improvement of oral health.4 It has been found to have antimicrobial health benefits and antioxidant properties.5 There are also suggestions that tea extracts offer protection against bone loss,4 body weight control, anti-hypertensive properties, solar ultraviolet protection, neuroprotective properties and anti-fibrotic properties.5 Therefore, tea provides a very interesting beverage with potential for a variety of medicinal uses and health-promoting benefits.
The use of traditional medicine is widespread, and plants have more potential for natural antioxidants that may serve as leads for the development of novel drugs. Thus, medicinal plant research is a search for cheaper and more readily available alternative treatment as conventional therapy is becoming expensive.3
Members of the genus Moringa and Camellia are known to possess very strong biological activities and thus widely used in ethnobotany medicine and pharmacy. However, information on possible adverse effects of these plants on human health are scarce. Therefore, the study of medicinal plants helps to understand the possible adverse effects of these plant species and protect humans and animals from natural poisons.
Oxidative stress has been defined as a disturbance in the balance between the production of reactive oxygen species (ROS) or free radicals and antioxidant defenses.6
Many metabolic reactions depend on oxygen7 –a primary oxidant in metabolic reactions designed to obtain energy from the oxidation of a variety of organic molecules──which is also used as substrate by numerous enzymes each with differing substrate affinities for oxygen and involved in a wide range of metabolic processes such as metabolism of amines, prostaglandins, purines, steroids, and amino acids.8 ROS are generated a by-product of normal aerobic metabolism, but their level increases under stress which proves to be a basic health hazard.9 These ROS include superoxide (O2 -), hydroxyl radical (HO.) and non-radical molecules like hydrogen peroxide (H2 O2 ). Nitric oxide (NO) is another abundant reactive radical that acts as an important oxidative biological signal in a large variety of diverse physiological processes, including smooth muscle relaxation, neurotransmission, and immune regulation.10
The mitochondrion is the major cell organelle responsible for ROS production.11 In order to protect the cell and organs systems of the body against reactive oxygen species, humans and other organisms have evolved a highly sophisticated and complex antioxidant protection system which involves a variety of components that are both endogenous and exogenous in origin that function interactively and synergistically to neutralize free radicals.12,13
The common antioxidant enzymes are superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase which catalyze free radical scavenging reactions. The nutrient derived antioxidants are ascorbic acid (vitamin C), vitamin E, carotenoids, and other low molecular weight compounds such as glutathione and lipoic acid as well as numerous other antioxidant phytochemicals present in a wide variety of plant foods.13,14
ROS can be beneficial, as they are used by the immune system as a way to attack and kill pathogens.12,15 Short-term oxidative stress may also be important in prevention of aging by induction of a process named mitohormesis.16
During the past decade, there has been a growing interest in the medical implications of free radicals. As a result of aerobic life, organisms must deal with the continuous generation of reactive oxygen species (O2 -, H2 O2 , •OH) as by-products of metabolism and defend itself against the harm that these can do to cellular macromolecules.12 Free radicals produced as a result of normal biochemical reactions in the body are implicated in contributing to a number of medical conditions such as cancer, aging, diabetes, atherosclerosis, immuno-supression and neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease.17,18
MATERIALS AND METHODS
Sample Collection and Preparation
Moringa oleifera (MO) leaves were obtained from ogbete main market in Enugu, Nigeria while popularly branded Camellia sinensis green tea (GT) from Qualitea, Ceylon Ltd., Sri Lanka was purchased from a supermarket in Enugu. Moringa leaf was identified and authenticated by Mr. Alfred Ozioko of Bio resources Development and Conservation Program (BDCP), Nsukka. Both samples were dried at room temperature and powdered by using an electrical blender.
100 g of dried, ground sample materials were soaked in 80% methanol (1L) for 5 days separately. The soaked material was stirred every 18 hours using a sterilized glass rod. The final extracts were passed through Whatman filter paper No.1. The filtrates obtained were concentrated under vacuum to dryness using a rotary evaporator at 40° C. The resulting fine powders obtained were weighed (MO=6.44 mg and GT=8.15 mg), dissolved in 300 ml of distilled water separately and stored at 4° C till use.
Assays and Principles
Total flavonoids were determined using the colorimetric assay developed by Zhishen et al.19 An aliquot (1 ml) of extract (concentration 1 mg/ml) was added to 10 ml volumetric flask containing 4 ml of distilled H2 O. Into the flask, 0.3 ml 5% NaNO2 was added and 5 min later 0.3 ml 10% AlCl3 was added. After 6 min, 2 ml of 1M NaOH solution was added and the total volume was made up to 10 ml with distilled H2 O. The solution was well mixed and the absorbance was measured at 510 nm against the control that had been prepared in the same manner only with replacing the extract with distilled water. Total flavonoids content was expressed as mg rutin equivalents (RE) per g of dry extract.
Total phenols were determined by Folin-Ciocalteu reagent method.20 A dilute extract of each plant extract (0.5 ml of 1 mg/ml) or gallic acid (standard phenolic compound) was mixed with FolinCiocalteu reagent (2.5 ml, 1:10 diluted with distilled water) and aqueous NaHCO3 (2 ml, 7.5%). The mixtures were allowed to stand for 15 min and the total phenols were determined by colorimetry at 765 nm. The standard curve was prepared using 0, 6.25, 12.5, 25, 50, 100 mg/L (µg/ml) solutions of gallic acid (GA) in methanol: water (50:50, v/v). Total phenol values are expressed in terms of gallic acid equivalent (mg/g of dry mass), which is a common reference compound.
The reducing power of extracts of MO and GT was determined according to the method of Oyaizu.21 Various concentrations of extracts (6.25, 12.5, 25, 50,100 mg/ml of distilled water) were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide [K3 Fe(CN)6 ] (1%, w/v), and then the mixture was incubated at 50° C for 20 min. Afterwards, 2.5 ml of trichloroacetic acid (10%, w/v) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of upper-layer solution was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (0.1%, w/v), and the absorbance was measured at 700 nm. Ascorbic acid (AA) was used as standard antioxidant. Higher absorbance of the reaction mixture indicated greater reducing power.
Nitric oxide was generated from sodium nitroprusside and measured by the Griess Illosvoy reaction. Sodium nitroprusside in aqueous solution at physiological pH (7.2) spontaneously generates nitric oxide,21,22 which interacts with oxygen to produce nitrite ions that can be estimated using Greiss reagent. Scavengers of nitric oxide compete with oxygen leading to reduced production of nitric oxide.22 Two ml Sodium nitroprusside (10 mM) in 0.5 ml phosphate-buffered saline (pH 7.4) was mixed with 0.5 ml of different concentrations of the extracts dissolved in the suitable solvent systems (distilled water) and incubated at 25° C for 150 min. The samples from the above were reacted with Greiss reagent (1% sulphanilamide, 2% H3 PO4 and 0.1% napthylethylenediaminedihydrochloride) in ratio 1:1. After incubation at room temperature for 30 mins, the absorbance of the chromaphore formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with napthylethylenediamme was read at 540 nm. Control experiment without the test compounds but with equivalent amount of buffer was conducted in an identical manner.
Calculated the % inhibition by formula and plot graph in comparison.
Formula:
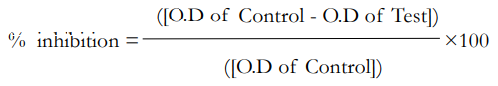
RESULTS
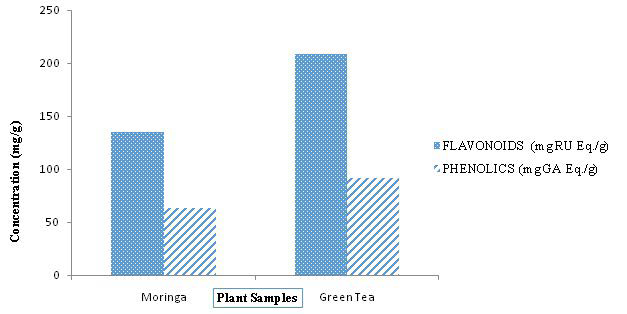
Phytochemical analysis of Moringa oleifera (MO) and green tea (GT) shows that total flavonoid concentration in both plants is 135.14±5.20 mg/g and 208.24±14.38 mg/g rutin equivalent (RE) respectively (Table 1). The total phenolic concentration in MO and GT extracts are 62.85±1.70 mg/g and 91.68±0.22 mg/g Gallic acid equivalent (GA Eq) respectively (Table 1). Administration of monosodium glutamate (MSG).
| Table 1. Flavonoid and Total Phenolics Concentration of MO and GT (mg/g) |
|
Sample
|
MO |
GT
|
|
Flavonoids
|
135.14 ± 5.20 |
208.24 ± 14.38 |
| Total Phenolics |
62.85 ± 1.70 |
91.68 ± 0.22
|
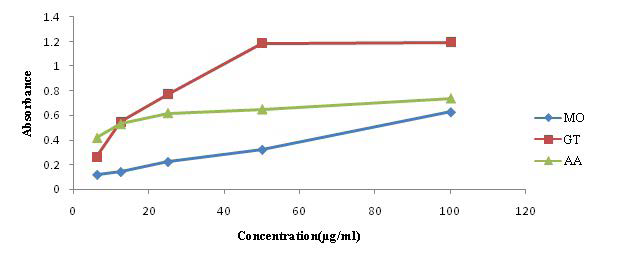
Table 2 presents the result of reducing power assay for the plants compared to ascorbic acid (AA), a known antioxidant used as standard. The result showed dose-dependent reduction ability with a maximum absorbance of 0.629 and 1.195 for Moringa and green tea respectively, at a concentration of 100 µg of the extracts.
| Table 2. Dose-dependent Reducing Power of Moringa, Green Tea and Ascorbic Acid |
|
Concentration (µg/ml)
|
MO |
GT |
AA |
| 6.25 |
0.120 ± 0.043 |
0.263 ± 0.032 |
0.421 ± 0.003
|
|
12.50
|
0.143 ±0.031 |
0.547 ± 0.021 |
0.532 ± 0.059 |
| 25.00 |
0.225 ± 0.010 |
0.771 ± 0.057 |
0.620 ± 0.013
|
|
50.00
|
0.324 ± 0.111 |
1.188 ± 0.011 |
0.651 ± 0.004 |
| 100.00 |
0.629 ± 0.022 |
1.195 ± 0.067 |
0.738 ± 0.023
|
| Means±SD of three independent determinations, difference is not significant (p>0.05) |
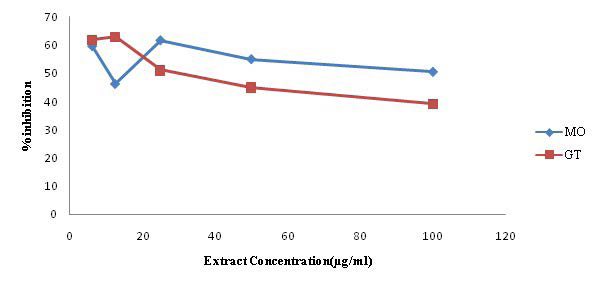
The result of the nitric oxide generation and Scavenging assay is presented in Table 3. A marked decrease in the absorbance at various concentrations of the extracts as against the control shows a good nitric oxide scavenging activity. GT appears to have a better percentage (61.82%) inhibition of nitric oxide at lower concentration (6.25 µg/ml) compared to MO (61.57%) which requires a higher concentration of the extract (25 µg/ml).
DISCUSSION
Flavonoids and Total Phenolics
Over the past years, numerous experimental and epidemiological studies have shown that a wide variety of phytochemicals such as flavonoids, phenolics, catechins are able to slow down oxidative stress-induced damage leading to large number of diseases.14 Natural products mainly from the plant kingdom among which we have Moringa oleifera and Camellia sinensis, offer a wide range of biologically active compounds that act as natural antioxidants with potential in drug discovery and development.14,19 Flavonoid and phenolic compounds are known for their potent antioxidant properties.
Various values of total flavonoid have been reported for both Moringa and Green tea. Tariq and Reyaz,23 reported a value of 14 mg/g for Camellia sinensis while Nor Qhairul Izzreen, and Mohd Fadzelly,24 reported a value of 20.9 mg/g for Camellia sinensis. A similar study in Nigeria25 reported total flavonoids in green tea as 10.0 mg/g. However, the total flavonoid content obtained in this study though higher [(135.14±5.20 and 208.24 ± 14.38 mg/g Rutin Equivalent (RU Eq)], falls within the range of 16.02-233.68 mg RU Eq/g reported by Stankovic et al.26 The total flavonoid concentration in Moringa is observed to be less than that in Camellia sinensis though the difference is not significant (p<0.05). Values ranging from 65.38±2.37 mg/g to 645.00±0.10 mg /g have been reported for total flavonoid in methanolic extracts of Moringa. 27 The results obtained in this study have shown that both Moriga and Green tea leaves are good source of total flavonoid.
The total phenolic concentration obtained in this study for Green tea extract is comparable to the findings of Nor QhairulIzzreen and Mohd Fadzelly,24 However, higher values have also been reported by other researchers.23,25,28
As with the total flavonoid, the total phenolic concentration [mg/g Gallic acid equivalent (GA Eq)] in Moringa (62.85±1.70 mg/g GA Eq) is less than that in Camellia sinensis (91.68±0.22 mg/g GA Eq). The total phenolic value obtained for Moringa in this study is at the same level with that reported by Woranan et al.29 Higher total phenolic values of 216.45±4.64 mg GA Eq/g and 541.00±0.02 mg GA Eq/g have also been reported for Moringa (Figure 1).27,30
Figure 1. Comparison of Flavonoids and Total Phenolics in Test Extracts
Reducing Power
Table 2 presents the result of reducing power assay for the plant extracts compared to ascorbic acid (AA), a known antioxidant used as standard. The result showed dose-dependent reduction ability with a maximum absorbance of 0.629 and 1.195 for Moringa and Green tea respectively, at a concentration of 100 µg of the extracts.
The reducing power of a substance or compound is related to its electron transfer ability and may therefore serve as a significant indicator of its antioxidant activity.31 The reducing ability of the methanolic extracts of Moringa and Green tea was measured by the transformation of Fe3 + to Fe2 + in the presence of the extract at 700 nm where increased absorbance of the reaction mixture indicates increased reducing power. Dose-dependent reduction ability was observed in the test extracts (Figure 2). This trend was similarly observed by Koruthu et al,21 and Nurul et al32 for Moringa and Tariq and Reyaz,33 for Green tea. The reducing power of green tea is higher than that of Moringa, although both Moringa and Green tea compare favorably with ascorbic acid (a standard antioxidant). However, Green tea has higher reducing power than ascorbic acid contrary to Tariq and Reyaz,33 while Moringa and ascorbic acid shows comparable reducing ability similar to the findings of Ogbunuga for et al.34 Thus, both plants may be a good source of antioxidants (Figure 2).
Figure 2. Reducing Activity of Plant Extracts Compared to Ascorbic Acid
Nitric Oxide Scavenging Activity
The result of the nitric oxide generation and Scavenging activity is presented in Table 3. GT appears to have a better nitric oxide scavenging activity (61.82% inhibition) at lower concentration (6.25 µg/ml), compared to MO (61.57% inhibition) which requires a higher concentration of the extract (25 µg/ml).
| Table 3. Nitric Oxide Generation and Scavenging Activity of Moringa and Green Tea (% inhibition) |
|
Concentration (µg/ml)
|
MO |
GT
|
|
6.25
|
59.52 |
61.82
|
|
12.50
|
46.18 |
62.88
|
|
25.00
|
61.57 |
51.14
|
|
50.00
|
54.92 |
45.08
|
|
100.00
|
50.44 |
39.28
|
Nitric oxide (NO) is an important bioregulatory molecule, which has a number of physiological effects including control of blood pressure, neural signal transduction, platelet function, antimicrobial and antitumor activity.35,36 It is a diatomic free radical that is extremely short-lived in biological systems.37 NO also shows toxic property after reaction with oxygen and superoxide radicals and is said to be involved in a number of important human diseases.38 The reaction products are able to cause much cellular damage. Thus, the unregulated production of nitric oxide can cause nitrosative stress, leading to damages of proteins/ DNA and to cell injury and death.39 NO scavenging capacity is determined by the decrease in the absorbance at 540 nm, induced by antioxidants. The result obtained in the in vitro NO assay suggests that the plant extracts have the capacity to scavenge NO as shown by high percentage (%) inhibition.40 Moringa showed a stronger tendency, based on the percentage of NO inhibited at various concentration compared to green tea. The results are comparable to the work of Jagetia et al (Figure 3).35
Figure 3. Effect of the Extracts on the in vitro Nitric Oxide Assay
CONCLUSION
This study confirmed the usefulness of the medicinal plants: Camellia sinensis, and Moringa oleifera. The present study showed that Moringa, and green tea plants have substantial amounts of flavonoids and total phenols which could play significant roles in the antioxidant properties of both plants.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.