INTRODUCTION
Cyanobacteria are photosynthetic Gram-negative prokaryotes, which are considered as one of the most ancient autotrophic forms of life on the planet. They are traditionally used as dietary and therapeutic agents in different parts of the world. Recently, cyanoprokaryotes have gained more scientific interest concerning their ability to produce compounds with antioxidant activity.1,2,3 As a phototrophic form of life, cyanoprokaryotes are under the influence of intensive oxidative stress caused by produced during photosynthesis free radicals. To prevent serious cellular damage these organisms have developed protective mechanisms based on the activity of antioxidative enzymes like superoxide dismutase and catalase, as well as low molecular weight antioxidants like glutathione, vitamins (ascorbic acid, tocopherols), phenols, microsporine amino acids and others. The antioxidant capacity of certain blue-green algae was compared with medicinal plants and it was found that cyanoprokaryotes are potent producers of different types of antioxidants.3,4,5 Recent studies have demonstrated a strong antioxidant activity of scytonemin, a ultraviolet (UV)-absorbing pigment, isolated from Nostoc commune.6 Antioxidative potential has been reported for extracts derived from different Nostoc species.1,4,5,7 The number of cyanoprokaryotes with reported antioxidant activity is increasing and includes species from the genera Nostoc, Spirulina, Fischerella, Phormidium, Anabaena, Oscillatoria, Calothrix.5,8 In addition, cyanobacteria produce a variety of low molecular weight compounds (lipopeptides, aminoacids, fatty acids, alkaloides, macrolides, phytosterols, amides, etc.) that have antitumor, antiviral, larvicidal, herbicidal, enzyme inhibition, antibacterial, antimycotic, cytotoxic or immunomodulatory activity.9 The cryptophicines are a typical example. They were initially isolated from a lichen symbiont belonging to the genus Nostoc.10 These compounds have a strong antiproliferative effect due to their ability to inhibit tubuline synthesis and induce mitotic spindle destruction. Other examples for biologically active substances derived from Nostoc species are the nostocyclopeptides with cytotoxic activity,11 nostocycline A that has antimicrobial effect12 or insulapeptolides,13 nostopeptines,14 microviridins G and H15 with protease inhibitory role. Another type of cyanobacterial paracyclophanes (nostocyclophanes) exhibit antibacterial, fungicidal and cytotoxic activities.16,17 Here we can include also nostocyclopeptide M1 that shows antitoxin activity against microcystins,18 lipopeptides with antibiotic effects like muscoride (isolated from Nostoc muscorum) and nostocine A (derived from Nostoc spongiaeforme).19,20 Indoles with antiviral and cytotoxic effects (for instance staurosporine and indolecarbazole), were isolated from Nostoc sphaericum.21 The protein cyanovirin, which is derived from Nostoc ellipsosporum, has antiviral activity and it is specifically effective against all human immunodeficiency viruses.22 It has been recently shown that an extract from Nostoc commune var. sphaeroides Kützing suppresses the expression and secretion of proinflammatory cytokines in murine macrophages and splenocytes and thus, exerts immunomodulatory activity.23 The significance of cyanobacteria (Nostoc species in particular) as a source of antioxidants and compounds with other biological activity stems from their diversity, accessibility and the possibility for large-scale cultivation in bioreactors.24
More than 60 marine and 20 freshwater cyanoprokaryotic species are identified as producers of toxins. Most of them belong to the genera Anabaena, Aphanizomenon, Nostoc, Microcystis, Nodularia, Cylindrospermopsis, Planktothrix, Oscillatoria, Raphidiopsis, Phormidium and Lyngbya.25,26 During the last two decades, many microcystin-producing Nostoc species have been identified.27,28,29,30 It has been suggested that cyanobacteria growing on sediments in or around water basins could produce a significant amount of microcystins31 and therefore their distribution should be monitored and routine tests for microcystins content should be performed for drinking water and water basins.27 Certain lichens associated with Nostoc sp. strain IO-102-1 produce 6 rare forms of microcystins.32 Except microcystins some Nostoc species produce other toxic compounds like nostocarboline and the neurotoxic aminoacid β-methylamino-L-alanine.33
To date, there is little information about the species Nostoc microscopicum concerning the production of biologically active compounds and cyanotoxins. This is the first report that demonstrates microcystins and saxitoxins production by Nostoc microscopicum. We also detected a moderate antioxidant activity of Nostoc microscopicum extracts and cytotoxic effect on three continuous human cell lines. These data provide a base for further investigations of Nostoc microscopicum aiming to identify novel biologically active compounds.
MATERIALS AND METHODS
Preparation of Nostoc Microscopicum Extracts
Four cyanobacterial extracts were obtained using different extracting agents (Table 1). Nostoc microscopicum Carmichael ex Bornet & Flahault (Culture Collection of Autotrophic Organisms (CCALA) #124) lyophilized dry mass was purchased from the CCALA, Institute of Botany, Academy of Sciences, Czech Republic. For the preparation of each extract 500 mg lyophilized material were mixed with 500 µL of the corresponding solvent. All four samples were incubated at room temperature in the dark for 24 h. After that the extracts were centrifuged for 15 minutes at 5000 rpm and the resulting supernatant was filtered through Whatman no 1 paper. Following filtration, the samples were freeze-dried and the obtained dry substances were dissolved in distilled water to a final concentration of 4 mg/mL. Distilled water or D-PBS (Gibco, Invitrogen, USA) were used for further dilution of the extracts during the experiments. All extract solutions were stored at 4 °С in a dark place.
| Table 1: Nostoc microscopicum extracts assayed. |
| Extract |
Abbreviation |
Solvent |
| Extract-1 |
E-1 |
5% acetic acid in Milli-Q water |
| Extract-2 |
E-2 |
75% methanol in Milli-Q water |
| Extract-3 |
E-3 |
75% ethanol in Milli-Q water |
| Extract-4 |
E-4 |
Dimethyl sulfoxide (DMSO) |
Determination of DPPH Free Radical Scavenging Activity (DPPH assay)
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay is based on a spectrophotometric detection of the change in 520 nm absorbance due to 2, 2-diphenil-1-picrylhydrazyl radical scavenging by the test substance.34 50 µL of 200 µM DPPH (Sigma-Aldrich, Germany) methanolic solution was mixed with 50 µL of Nostoc microscopicum extract. The reaction mixture was incubated at room temperature for a period of 1 h in the dark. All extracts were assayed in 3 different concentrations – 200 µg/mL, 100 µg/mL and 50 µg/mL. Ascorbic acid solution in serial dilutions (600 µg/mL, 400 µg/mL, 200 µg/mL, 100 µg/mL, 50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.25 µg/mL, 3.125 µg/mL, 1.6 µg/mL) was used as a standard. All samples were tested in triplicates. Following incubation with DPPH radical absorption at 520 nm was detected using Synergy 2 microplate reader (Biotek, USA). The following formula was used to calculate the percent antioxidant activity (DPPH radical scavenging activity):
% Antioxidant activity = [(Аblank-Аsample)/Аblank] × 100
where Аblank is the absorbance of the control that contains all reagents except the Nostoc microscopicum extract, and Аsample is the absorbance of the sample that contains Nostoc microscopicum extract.
Total Phenolic Content
The total phenolic content of Nostoc microscopicum extracts was estimated using the Folin-Ciocalteu method.35 100 µL of each extract were mixed with 1.8 mL 2% water solution of sodium carbonate and incubated for 2 minutes at room temperature. After that 100 µL 50% water solution Folin-Ciocalteu reagent was added to the mixture and all samples were incubated for 0.5-2 h at room temperature in the dark. As a result, colored complexes form in the positive samples that contain phenols. Gallic acid (Sigma-Aldrich, Germany) in serial dilutions (500 µg/mL, 250 µg/mL, 125 µg/mL, 62.5 µg/mL, 31.25 µg/mL, 15.625 µg/mL, 7.8 µg/mL, 3.9 µg/mL, 1.95 µg/mL, 0.98 µg/mL) was used as a standard. The results were obtained based on spectrophotometric detection of absorption at 760 nm. The data was presented as µg equivalent gallic acid/mg extract (µg GAE/mg). All samples were analyzed in triplicates.
ELISA for Detection of Saxitoxins
The saxitoxins content of N. microscopicum methanolic extract was evaluated using RidascreenTM kit (R-Bopharm, Germany) with a detection limit of 0.010 ppb (µg/L saxitoxins). The samples and the standards with known saxitoxins concentration were pipetted on a microtiter plate that contain coated with saxitoxins-specific antibodies wells. Then, they were mixed with enzyme-conjugated purified saxitoxins. Hence, the free saxitoxins in the samples and the enzyme-conjugated ones will compete for binding to the toxin-specific antibodies coated on the plate wells. Following 2 h incubation at room temperature, the plates were washed with buffer in order to remove the unbound enzyme-conjugated saxitoxins. A chromogenic substrate solution for the marker enzyme was added to the plate following washing. As a result, a colored product forms in the wells containing complexes of enzyme-conjugated saxitoxins and saxitoxins-specific antibodies. The reaction was detected spectrophotometrically, absorption at 450 nm wavelength was analyzed. The resulting values (absorption units) are reversely proportional to the concentration of saxitoxins in the sample.
Evaluation of Microcystins Concentration
Microcystins enzyme-linked immunosorbent assay (ELISA) kit (Abraxis LLC, USA) with detection limit 0.010 ppt (ng/L microcystins) was used to assess the microcystins content of Nostoc microscopicum methanolic extract. The assay is based on competitive ELISA and it is analogical to the described above saxitoxins ELISA.
In Vitro Cytotoxicity Assays
For evaluation of potential cytotoxic effects of Nostoc microscopicum extracts we used three continuous human cell lines: HeLa (ATCC CCL 2, NBIMCC 164) established from cervical adenocarcinoma cells; A549 (ATCC CCL 185, NBIMCC 2404) derived from lung carcinoma, and FL (ATCC CCL 62, NBIMCC 94) established from normal amniotic cells. The cells were expanded in 75 cm2 culture flasks in Dulbecco’s modified eagle’s medium (DMEM) (Gibco, Invitrogen, USA) supplemented with 10% heat-inactivated fetal bovine serum (PAA Laboratories, Austria) and antibiotics (100 U/mL penicillin and 100 µg/ml streptomycin (Sigma-Aldrich, Germany)). The cells were cultivated at 37 °С, 5% CO2 and high humidity until they reach confluence. Then, they were trypsinized and the concentration of viable cells was determined using the Trypan blue assay. A549, FL and HeLa cells were diluted to a concentration of 2×105 cells/ml and seeded in 96-well plates (200 µL cell suspension/well). After 24 h incubation, Nostoc microscopicum extracts in 4 different concentrations (400 µg/mL, 200 µg/mL, 100 µg/mL, 50 µg/mL) were added to the culture plates and the cells were cultured for 24 h, 48 h and 72 h. An equivalent amount of dulbecco’s phosphate buffered saline (D-PBS) (Gibco, Invitrogen, USA) was added to the control wells with cells. All samples were tested in triplicates.
The cytotoxic effect of Nostoc microscopicum extracts was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT is a yellow colored tetrasolic salt that is reduced by mitochondrial dehydrogenases in viable cells to a purple colored formazan product, which accumulates in mitochondria. Dead and impaired cells cannot transform MTT. In our experiments, the MTT assay was performed according to the methodic of Edmondson.36 At the end of each exposure period (24, 48 and 72 h) 20 µL 5 mg/ml МТТ (Sigma-Aldrich, Germany) solution were added to the culture wells. Then, the plates were incubated for 3-4 hours in the dark at 37 °С, 5% CO2 and high humidity. After that, the culture medium was discarded and the cells were washed with D-PBS (Gibco, Invitrogen, USA). The accumulated formazan was solubilized with dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Germany): 100 µL DMSO per well were added and incubated with the cells at 37 °С for 15 minutes. The amount of formazan accumulated in the cells was measured photometrically at 570 nm on a synergy 2 microplate reader (Biotek, USA). The mean absorbance measured for the cells without any extract addition was referred as 100% cell viability (control).
Percentage of inhibition (PI) was calculated as follows:
PI (%) = [1-(A570 nmtest)/(A570 nmcontrol)]×100
IC50 values for each extract were then calculated.
Statistical Analysis
Results are presented as mean±SE from individual determinations with at least three replicates. Statistical differences were analyzed by the Mann–Whitney U-test using the Statview programme (SAS Institute, Inc.). Values of p<0.05 were considered as significant. All results were compared to those from the control group.
RESULTS
Antioxidant Activity of Nostoc Microscopicum Extracts
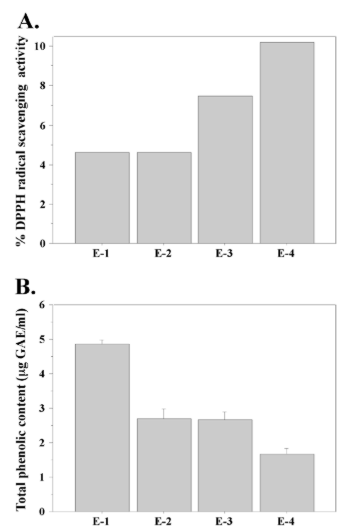
To evaluate the antioxidant activity of N. microscopicum extracts we used two of the most commonly performed methods—the DPPH radical scavenging activity assay and the Folin-Ciocalteu method that measures total phenolic content. Both methods are based on spectrophotometric measurement of the interaction between an antioxidant and a substance that changes its color due to alteration in redox potential.37 The DPPH assay detects changes in the absorption spectrum of the free radical DPPH when it is reduced by an antioxidant.34 Figure 1A shows the results from the DPPH assay with Nostoc microscopicum extracts. A clear difference in the DPPH radical scavenging activity was found between the extracts obtained with different solvents. The DMSO extract showed the highest antioxidant activity (more than 10%), while the extracts obtained with acetic acid and methanol displayed two times lower DPPH radical scavenging activity. Extract-3, where we used ethanol as extracting agent, showed median antioxidant activity compared to the other extracts.
Recent studies have shown that phenolic compounds contribute to the antioxidant activity of microalgae, including cyanobacteria.3,5 Therefore, in order to better characterize the antioxidant activity of N. microscopicum extracts we measured their total phenolic content. The results of this experiment are shown in Figure 1B. They demonstrate relatively low, but significant concentration of phenolic compounds in N. microscopicum extracts. Again, we found a difference between the four extracts. In this case, extract-1 showed the highest result with approximately 5 µg GAE/mL, while extract-4 had the lowest total phenolic content (lower than 2 µg GAE/mL). The level of phenolic compounds detected in the methanolic and ethanolic extracts was median compared to E-1 and E-4.
Figure 1: Antioxidative potential of Nostoc microscopicum extracts. A. Percentage (%) antioxidant activity based on measurement of DPPH free radical scavenging activity. B. Total phenolic content of Nostoc microscopicum extracts. Data represent mean ±SE.
Nostoc Microscopicum Produces Cyanotoxins
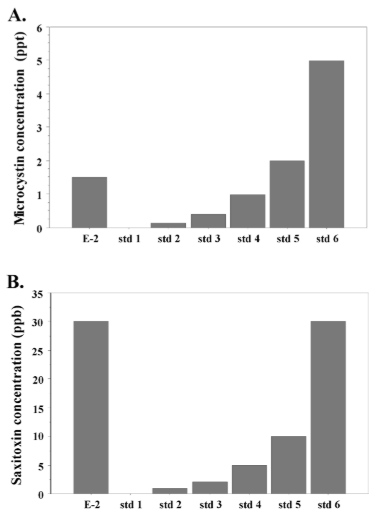
For assessment of microcystins and saxitoxins production we used only extract-2 because it has been shown that cyanotoxins are most effectively extracted by methanol.38 We detected presence of both microcystins and saxitoxins in the Nostoc microscopicum extract (Figures 2A and 2B). The measured microcystins concentration was 1.5 ppt (ng/L). Interestingly, the saxitoxin content was very high, equal to the standard with the highest concentration in our assay—about 30 ppb (30 µg/L). This result demonstrates that Nostoc microscopicum is a potent producer of saxitoxins.
Figure 2: Cyanotoxins production by Nostoc microscopicum. A. Concentration of microcystins (ng/L). B. Concentraction of saxitoxins (µg/L). E-2:
methanolic Nostoc microscopicum extract; std: standard.
In Vitro Cytotoxicity of Nostoc Microscopicum Extracts
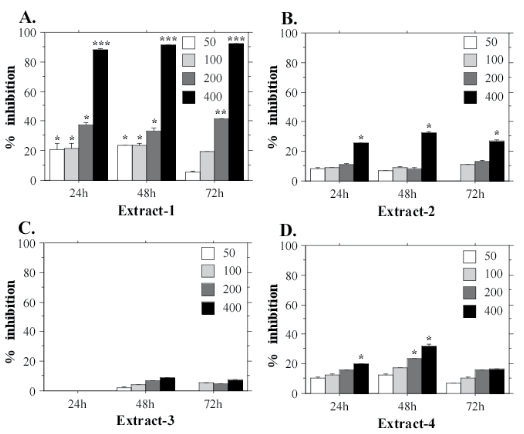
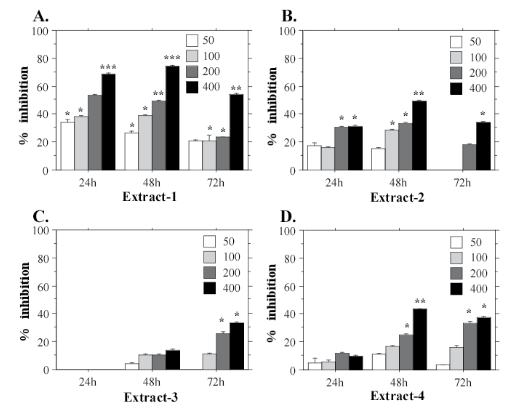
The potential cytotoxic effects of Nostoc microscopicum extracts were investigated using three human cell lines—two cancer cell lines (A549 and HeLa) and one cell line derived from normal amniotic cells (FL). The aim of our experiments was to determine whether Nostoc microscopicum extracts exert a common cytotoxic effect. The results from the MTT assays with FL cells are shown on Figure 3. They demonstrate that E-1 has the strongest cytotoxic effect with a mean IC50 value 253.1 µg/mL calculated for the 24 h test-period (Figure 4A). The extract obtained with ethanol (E-3) had the weakest effect on the cell viability. FL cells demonstrated clear dose-dependent response to all extracts with a prominent cytotoxic effect at the highest tested concentrations (200 and 400 µg/mL). The same tendency was determined for HeLa (Figure 4) and A549 cells (Figure 5).
Figure 3: Cytotoxic effects of different concentrations (50, 100, 200, 400 µg/mL) of Nostoc microscopicum extracts on FL cells after exposure for 24, 48 and 72 h determined with the MTT assay. Data are reported as mean values±SE from individual determinations with at least three replicates. Asterisks indicate significant differences in percentage of inhibition compared to the control (* p<0.05; **p<0.01; ***p<0.001).
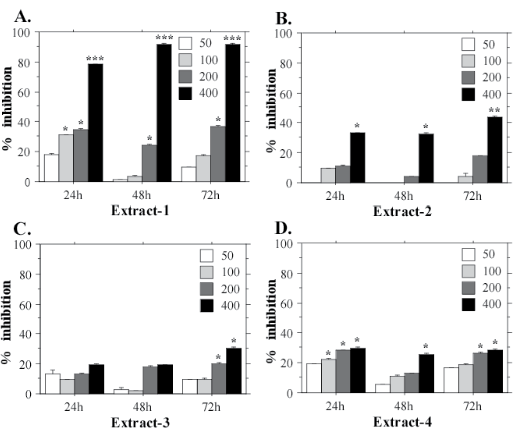
The MTT assays with HeLa cells confirmed that N. microscopicum extract-1 has the highest cytotoxic activity (Figure 4A). The mean IC50 value for HeLa cell line treated with E-1 for 24 h is 269.7 µg/mL. Extract-2 also showed a prominent inhibition of the cell functions (Figure 4B) at the highest test-concentration (400 µg/mL).
Figure 4: Cytotoxic effects of different concentrations (50, 100, 200, 400 µg/mL) of Nostoc microscopicum extracts on HeLa cells after exposure for 24, 48 and 72 h determined with the MTT assay. Data are reported as mean values±SE from individual determinations with at least three replicates. Asterisks indicate significant differences in percentage of inhibition compared to the control (*p<0.05; **p<0.01; ***p<0.001).
Similar to HeLa and FL, А549 cells displayed the strongest inhibition of the mitochondrial dehydrogenases when treated with extract-1 (Figure 5). This cell line also demonstrated highest sensitivity to E-1 with significantly lower IC50 value at the 24 h test-period (173.3 µg/mL) at 400 µg/mL. E-1 exhibited the highest cytotoxic effect during the first 24 h of exposition, while at the longer exposition times (48 h, and especially 72 h) the inhibition effect was weaker (Figure 5A). On the contrary, extract-3, which generally exhibits the weakest cytotoxic activity, shows its effect after a longer exposition period, i.e. 72 h (Figure 5C). This tendency is evident also for HeLa cells (Figure 4C), which suggests a specific mechanism of action for Е-3 on the cancer cell lines. However, more experiments are needed to confirm this hypothesis.
Figure 5: Cytotoxic effects of different concentrations (50, 100, 200, 400 µg/mL) of Nostoc microscopicum extracts on A549 cells after exposure for 24, 48 and 72 h determined with the MTT assay. Data are reported as mean values±SE from individual determinations with at least three replicates. Asterisks indicate significant differences in percentage of inhibition compared to the control (*p<0.05; **p<0.01; ***p<0.001).
DISCUSSION
Over recent decades scientists and industrialists have concentrated their interests on natural antioxidants. The reason is that synthetic antioxidants like butylated hydroxyanisole (BHA), butylated hydroxytoluene (ВНТ), tert-butylhydroquinone (TBHQ) and propyl gallate (PG) have been shown to manifest carcinogenic and toxic effects in experiments with laboratory animals.39 Microalgae, in particular cyanoprokaryotes, have been suggested as a very convenient source of antioxidants.9 They are phototrophic organisms and thus, they have developed effective antioxidant systems that protect them from the free radicals produced during photosynthesis. Microalgae could be easily grown in a laboratory and used for large-scale cultivation in bioreactors with the ability to control the quality of the cultures by providing purified culture medium that is free of pesticides, herbicides and other toxic substances. Therefore, microalgae provide a more accessible way to produce qualitative substances of interest.24
Several research groups have recently published data showing antioxidative potential of extracts derived from different Nostoc species,5,7,40 as well as production of specific antioxidants.6 To date, there are no reports on Nostoc microscopicum concerning antioxidative potential or manifestation of other biological activities. Ivanova et al41 have demonstrated different genotoxic effects of Nostoc microscopicum extracts on Allium cepa. Hrouzek et al42 report a weak cytotoxic effect of Nostoc microscopicum extract on mouse cell lines YAC-1 and Sp/2. However, there is no information about potential toxic effects of this cyanoprokaryotic species on human cell lines. The lack of data about Nostoc microscopicum and the numerous reports on diverse biological activities of extracts or purified substances from many Nostoc species stimulated our investigations on the antioxidative potential, cytotoxic effects and cyanotoxins content of N. microscopicum extracts.
The examined N. microscopicum extracts showed differences in antioxidant activity. They differed in DPPH free radical scavenging activity and in total phenolic content. Presumably, the main reason for this variation is the type of solvent used for extraction. All extracting agents, namely acetic acid, methanol, ethanol and DMSO belong to the group of polar solvents. Among them, only DMSO is aprotic polar solvent, while the others are protic solvents. Protic and aprotic polar substances differ in mechanism of action. Aprotic solvents do not form hydrogen bonds between dissolved molecules and thus, provide relatively free and more reactive nucleophiles in the solution. Protic solvents like water form hydrogen bonds. They could play a role of weak nucleophiles that form bonds with weak electrophiles.43 Our results indicate that protic polar solvents, in particular DMSO, effectively extract non-phenolic compounds with antioxidant activity.
It has been suggested that phenolic compounds display antioxidant activity in microalgae. Recently, it was found that total phenolic content in microalgae biomass increases after exposition to UV light, which shows that these compounds participate in the antioxidant response against the stress induced by UV light.44 In addition, there are reports that compared the antioxidant activity and total phenolic content of extracts from different microalgal species. They clearly demonstrate that the extracts with high phenolic content have also high antioxidant activity.45,46 Goiris et al3 demonstrated that phenolic compounds play a significant role for the antioxidant activity of microalgae. Interestingly, in our experiments we found a reversed correlation between the total phenolic content of Nostoc microscopicum extracts and the results from the DPPH assay. The extract with the lowest DPPH radical scavenging activity displayed the highest total phenolic content compared to the other Nostoc microscopicum extracts. On the other hand, the extract with highest DPPH radical scavenging activity had the lowest total phenolic content. Probably, these surprising results are based on the characteristics of the assays we used. The Folin-Ciocalteu method detects only a particular group of compounds, i.e. phenolic compounds, while the DPPH assay measures the antioxidant activity of a broader spectrum of substances, including phenols, carotenoids, polysaccharides, polyvalent unsaturated fatty acids, tocopherols and others.47 Another possible explanation is that the DMSO used as solvent for preparation of extract-4 does not extract effectively phenolic compounds.
Numerous reports have shown that certain widely distributed members of genus Nostoc produce microcystins.27,28,29,30,32 Sivonen et al48 reported that Nostoc sp. strain 152 produces 9 hepatotoxic peptides with similar toxicological properties to the hepatotoxic hepta- and pentapeptides produced by other cyanobacterial species. Five of these peptides were identified as novel types of microcystins – LR homologues. Some lichens associated with terrestrial Nostoc sp. strain IO-102-1 also produce six rare forms of microcystins.32 Nostoc species produce other toxic compounds except microcystins. Some Nostoc root symbionts of sago palms (Cycas micronesica) produce β-methylamino-L-alanine33 – a neurotoxic amino acid, which plays a role in amyotrophic lateral sclerosis/parkinsonism-dementia. These data stimulate the studies on microcystins production by Nostoc microscopicum.
Saxitoxins are the main component of a paralytic poison, known as paralytic shellfish poison (PSP). They block the Na+ channels in the membranes of nerve and muscle cells resulting in paralyses. A dose of 1-3 mg saxitoxins is lethal for the human and ingestion of 0.5-1 µg could induce deafness and suffocation. Therefore, it is necessary to routinely monitor water basins and foods for saxitoxins content. Unlike microcystins, the information about saxitoxins production by Nostoc species is scarce. Teneva et al40 reported that Nostoc linkia and Nostoc punctiforme synthesize low amounts of saxitoxins. The present report demonstrates for the first time significant production of saxitoxins by Nostoc microscopicum.
The cytotoxic effect of Nostoc microscopicum extracts was examined at three exposure periods – 24, 48 and 72 h. The results we obtained show clear dose-dependent responses. The viability of the cells and their functions were inhibited with increasing the concentration of Nostoc microscopicum extract. This trend was evident for all three human cell lines treated with cyanobacterial extract – a result that shows a common cytotoxic effect, but not selective antitumor growth inhibition. This common cytotoxic effect could be due in part to the cyanotoxins content in the extracts. We cannot exclude that the toxic effect is induced by novel unidentified compounds, specifically produced by Nostoc microscopicum.
CONCLUSIONS
This is the first report that demonstrates cyanotoxins production by Nostoc microscopicum. In particular, we show a significantly high content of saxitoxins in Nostoc microscopicum extracts. These data warns for special attention and careful purification if this cyanobacterial species is used for manufacture of pharmaceutical and other industrial products. In addition, we report a moderate antioxidative potential of Nostoc microscopicum extracts, as well as cytotoxic activity against human cell lines. Collectively, our data contribute for a more detailed characterization of Nostoc microscopicum and stimulates further toxicological research on this cyanoprokaryote and research aiming to identify novel biologically active compounds with potential biotechnological applications.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.










