INTRODUCTION
Diabetes mellitus (DM) has been more prevalent across the world according to the epidemiological report of International Diabetes Federation (IDF).1 Among them, undiagnosed DM (UDM) in adult is gradually increasing in developed and developing countries.2 American Diabetes Association (ADA) proposed the latest guideline in Jan 2022, which is the Standards of Medical Care in Diabetes-2022.3 For the diabetic therapy, nutritional treatment would be the fundamental strategy including low carbohydrate diet (LCD).4 Clinical efficacy of LCD has been gradually recognized for improving glucose variability and weight reduction.5 Authors and collaborators have continued diabetic research and practice for long.6 Their clinical fields include LCD, continuous glucose monitoring (CGM), Glucagon-Like Peptide 1 receptor agonist (GLP-1RA), meal tolerance test (MTT) and others.7,8
Concerning LCD investigation, historical changes for nutritional therapy were observed. Calorie restriction (CR) was the standard method for diabetic diet therapy in previous period. After that, LCD method was introduced to medical and health care field by Atkins et al.9 LCD was evaluated to be clinically effective by Dietary Intervention Randomized Controlled Trial (DIRECT) study.10 Successively, LCD was generally defined as related with several diet methods.11 On the other hand, authors et al. initiated LCD in Japan and developed LCD medically and socially through the activity of Japan LCD Promotion Association (JLCDPA).12 Among our various activities, three representative methods of LCD were widely informed to general people. They are petite LCD, standard LCD and super LCD.13 These LCD nutritional options were applied to many obesity patients, and the results showed that 25% subjects achieved >10% of weight reduction.14
As to MTT, standardized liquid meal was ingested and assessed for β-cell function in diabetic patients.15 Similar to MTT, widely applied popular method includes oral glucose tolerance test (OGTT).16 Authors have developed MTT research, and proposed the simple method using breakfast.17 The standard meal is made from Japanese style standard breakfast including 70 g of carbohydrate.18 Applying this meal, the responses of blood glucose, insulin and C-peptide were investigated.19
By combination of the research of LCD and MTT, we have continued clinical diabetic studies. Among several patients with type 2 DM (T2DM), we have experienced a case of impressive characteristic aspects. In this case report, general outline of clinical course and treatment will be introduced.
CASE PRESENTATION
Family and Past History
The current case is 61-year-old man. Regarding his family history, there was unremarkable disease involved, such as diabetes mellitus, hypertension, coronary heart disease (CHD), cerebral vascular accident (CVA) or malignant disease.
For his previous medical history, he was born without any health problem. When he was born, his weight was 3500 g. It was much heavier than average baby weight. Her mother was not diabetic or did not have any medical problems during pregnancy. He did not have significant disease or impaired function until 54-yearsold.
Medical History and Status
The current case is 61-year-old T2DM male patient who has diabetic history of 4-years. He has become gradually rather obese after 55 years old and then he was diagnosed for T2DM. Then he was advised to reduce the weight, and he tried to continue mild LCD for a few years. He received 75 g OGTT in May 2020, in which it showed delayed pattern of glucose and insulin. The HbA1c value maintained about 6.1-6.4% for a few years. During summer 2021, he felt general malaise and weight loss and then received medical check. Consequently, he was pointed out to have exacerbated T2DM and to start strict LCD at once. Consequently, he and his wife have started to evaluated carbohydrate amount of his daily meal. Consequently, HbA1c and blood glucose decreased, associated with lower post-prandial blood glucose.
His physical status showed unremarkable findings, including consciousness, vital signs, lung, heart, abdomen and neurological examination. His stature, weight and body mass index (BMI) was 181 cm, 89 kg and 27.2 kg/m2, respectively. His laboratory examinations revealed that HDL-C 65 mg/dL, LDL-C 142 mg/dL, TG 100 mg/dL, GOT 22 U/L, GPT 28 U/L, r-GTP 25 U/L, BUN 17 mg/dL, Cr 0.8 mg/dL, UA 3.9 mg/dL, WBC 8600 /μL, RBC 508×106 /μL, Hb 16.2 g/dL, Plt 21.1×104 /μL, CRP 0.02 mg/dL. Urinalysis showed pH 6.0, protein (-), glucose (+), urobilinogen (+/-), occult blood (-) and ketone body (+). Other tests showed negative for chest X-P and electrocardiogram (ECG). Pulse wave velocity (PWV) test showed normal range results, where ankle brachial index (ABI) was 1.08/1.09 and cardio-ankle vascular index (CAVI) 10.0/10.0 for right/left.
Clinical Course
This case has applied strict LCD that has been known as superLCD method. It means that carbohydrate ratio in calorie calculation would be 12%. He actually continued to decrease carbohydrate as possible.20 Then, his daily profile of blood glucose became normalized and HbA1c values decreased from 7.8% to 6.3% for 3-months. He felt no remarkable complaints, symptoms, signs or abnormal biomedical exams during 4-months. During his clinical course, he received 75 g OGTT and continued taking daily meal data by digital camera associated with calculating carbohydrate amount and calorie included. Especially, 45-minutes post-prandial glucose of dinner was continued to check for months.21 Furthermore, the insulin response was investigated for endocrinological stimulus by glucagon test.
RESULTS
During the period of LCD, several data such as carbohydrate amount and calorie were checked and analyzed (Table 1). Table 1 revealed Western and Japanese styles of dinner provided. Blood glucose value was measured as 45-minutes post-prandial point of each dinner. Further, relationship between carbohydrate amount and 45-minutes post-prandial blood glucose was investigated, which was analyzed in Figure 1. It shows significant relationship between them. The formula for the regression curve is shown, which was y=1.9084x+104.76, R²=0.5789, p<0.05.
Figure 1. Relationship between Carbohydrate Amount in the Dinner and 45-minutes Post-prandial Blood Glucose
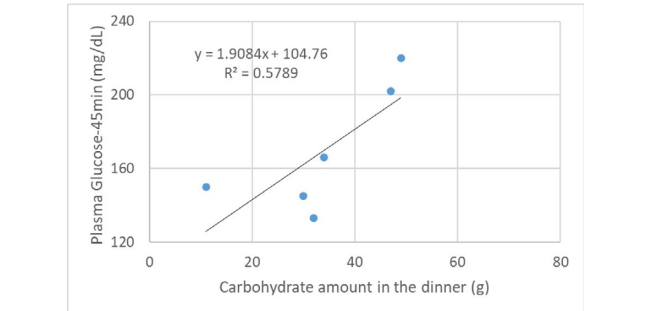
Table 1: Dinner Content with Carbohydrate Amount and Post-Prandial Blood Glucose
| Dinner |
Ingredient |
Each Carbo (gram) |
Total Carbo (gram) |
Total Calorie (kcal) |
A |
Tempura |
21.2 |
48.2
|
656
|
| Fish |
4.0 |
| White rice |
19.0 |
| Miso soup, etc. |
4.0 |
B |
Fried chicken |
28.0 |
33.4
|
652
|
| Cauliflower |
0.1 |
| Vegetables |
0.1 |
| Miso soup, etc. |
5.4 |
C |
Hot pot shabu-shabu |
0.1 |
10.8
|
445
|
| Beef |
0.3 |
| Tofu |
0.4 |
| Vegetables |
10.0 |
D |
Chili pepper soup |
7.9 |
30.0
|
205
|
| Pork |
0.2 |
| White radish |
2.4 |
| Vegetables |
19.5 |
Regarding the ability of insulin secretion, 75 g OGTT was conducted (Figure 2). Figure 2 includes totally four results of 75 g OGTT, in which Figures 2a and 2b are from the first author of current T2DM case in May 2020 and December 2021 (case 1), Figure 2c is from the second author without any diseases (case 2) and Figure 2d is from the third author with T2DM (case 3). Case 1 showed delayed response of glucose and immuno-reactive insulin (IRI) for T2DM. Case 2 showed normal response of glucose and IRI for normal subject. Case 3 showed hyperglycemia in which glucose increased to 240 mg/dL in 120-minutes. Related to 4 data of Figure 2, detail biomarkers of diabetes mellitus were summarized associated with detail background of case 1-3 in Table 2. Case 1 showed elevated fasting IRI, homeostasis model assessment insulin resistance (HOMA-R) in May 2020 and almost normal range of IRI and HOMA-R in December 2021. His insulinogenic index (IGI) values remained low during the period. Case 2 is 65-yearold physician and showed normal range of all biomarkers. Case 3 is 56-year-old nurse and showed normal range of HOMA-R and Homeostatic model assessment of β-cell function (HOMA-β), and decreased IGI. For detection of insulin secretion, glucagon stimulation test (GST) was conducted. As a result, c-peptide value increased from 2.0 ng/mL to 4.7 ng/mL during 6-minutes (Figure 3).
Figure 2. Responses of Blood Glucose and IRI in 75 g OGTT for Case 1-3
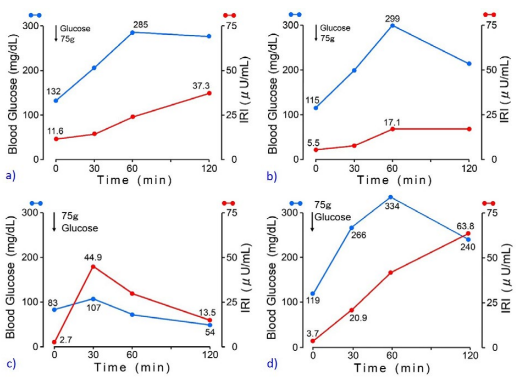
Case 1 is the first author who works in the administration section of the hospital.
Case 2 is the second author who specializes diabetes mellitus as a physician.
Case 3 is the third author who has worked in the outclinic as a hospital nurse.
Figure 3. Response of C-peptide for Glucagon Stimulation Test
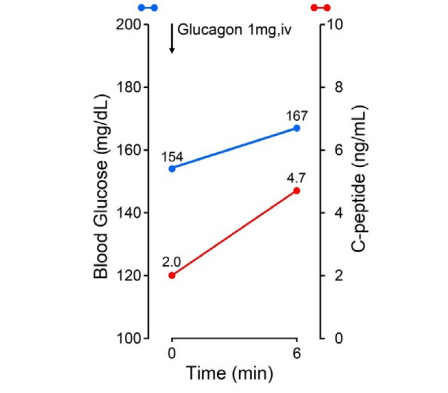
Table 2: General Background, HOMA Values and IGI in Three Cases
|
Case
|
Age/ Sex
|
Diagnosis
|
Test date (Mo/Year)
|
FPG (mg/dL)
|
IRI (µIU/L)
|
HOMA-R |
HOMA-13 (%) |
IGI |
Figure
|
|
Case 1
|
61 M
|
T2DM
|
May 2020
|
132
|
11.6
|
3.78
|
60.5
|
0.04
|
Figure 2a
|
|
Case 1
|
61 M
|
T2DM
|
Dec 2021
|
115
|
5.5
|
1.56
|
38.1
|
0.03
|
Figure 2b
|
|
Case 2
|
65 M
|
Normal
|
Jan 2022
|
83
|
2.7
|
0.55
|
48 .6
|
1.76
|
Figure 2c
|
|
Case 3
|
56 F
|
T2DM
|
Feb 2022
|
119
|
3.7
|
1.09
|
23.8
|
0.12
|
Figure 2d
|
| Case 1 is the first author who works in the administration section of the hospital.
Case 2 is the second author who specializes diabetes mellitus as a physician.
Case 3 is the third author who has worked in the out clinic as a hospital nurse. |
DISCUSSION AND CONCLUSION
The current case has T2DM, and HbA1c value has been elevated for years. Regarding the pathophysiological aspect, both of insulin resistance and insulin deficiency has been present to some degree from his history and biomedical examination results. BMI is currently 27.2 kg/m2, but once it weighed 100 kg with 30.5 kg/m2 of BMI. In this case report, detail investigation for several aspects is conducted, which are summarized in tables and figures. Discussion and perspectives would be described in this order.
In this case, super low carbohydrate diet (super-LCD) was applied and continued.13 It includes 12% of carbohydrate in calorie ratio, associated with normalized HbA1c and glycemic fluctuation. Along with super-LCD, he successfully continued meal diary every day and blood glucose profile perfectly (Table 1). We have continued to research and clinically apply LCDs for long. Among them, the clinical effect of LCD was found in many patients, and the cases of remarkable weight loss were also observed.20 There are actually three methods for LCDs, which are petite-LCD, standardLCD and super-LCD. The carbohydrate content in calorie ratio is 12%, 26% and 40%, respectively.13 From recent reports on LCD, various beneficial effects have been observed.22
For evaluating clinical LCD trials, carbohydrate ratio in the meal has been on discussion.17 A standard classification shows that high-carbohydrate diet (>45%), moderate carbohydrate diet (26-45%), LCD (10-25%), and very low-carbohydrate ketogenic diet (VLCKD) (<10%).18,19 As carbohydrate ratio is lower, glucoselowering efficacy is larger for T2DM.20 However, it has been not clear whether more carbohydrate restriction can bring the cardiovascular risk reduction or not.19
Meal tolerance test (MTT) was performed in advance to examine post-prandial blood glucose 30-minutes, 45-minutes, and 60-minutes for the case. As a result, 45-minutes post-prandial glucose was the highest.21 Then, post-prandial timing was decided 45-minutes for this case. Significant correlation was found between carbohydrate amount and 45-minutes post-prandial glucose in the dinner (Figure 1). We can calculate using the regression curve. When 20 g of carbohydrate is input, glucose value becomes 142 mg/dL, and when 50 g is input, it becomes 199 mg/dL. The measurement seems to be more or less consistent with actual carbohydrate intake and post-prandial glucose in T2DM patients.18 MTT and OGTT are often compared for simple and convenient in the clinical practice.23 Both are suitable methods to evaluate physiological β-cell ability for long-term.24 Comparative investigation revealed that MTT showed stronger response of β-cell than OGTT,24 and represented reproducible and reliable way of analyzing biomarkers of β-cell function.25
OGTT exams were checked twice, which were compared between in May 2020 and December 2021 (Figure 2). His weight was 95 kg vs 88 kg and his fasting IRI was 11.6 μIU/mL vs 5.5 μIU/mL. From these data, the degree of insulin resistance may be reduced. The pattern of glycemic and IRI for 75 g OGTT seems to be similar, and unremarkable change in insulin secretory reserve was observed. As a comparative control, case 2 showed rather low glucose on 120-minutes. Case 2 is healthy physician and usually on standard LCD, which may be involved in lower glucose on 120-min after 75 g OGTT. Case 3 has T2DM for more than 15-years, and is currently on petite LCD for better glucose variability. It showed delayed insulin response and blood glucose increased from 119 mg/dL to 334 mg/dL (215 mg/dL increase). From pathophysiological point of view, T2DM patient is reported to show 3 mg/ dL of glucose increase per 1 g intake of carbohydrate. When calculating as 75 g×3=225, this result seems to be similar to actual data.21 OGTT has been widely used for investigating glucose tolerance and insulin secretion in clinical practice.26 Estimation of β cell function by OGTT shows more physiological responses of glucose and insulin than those of frequently sampled intravenous glucose tolerance tests (FSIVGTT) and hyperglycemic clamp.27
HOMA-R, HOMA-β and IGI in case 1 suggested that insulin resistance was slightly reduced for 19-months, but insulin secretory capacity was similar (Table 2). Regarding the pathogenesis of all types of DM, increased insulin resistance and reduced insulin secretion would play a major role.26 It is rather difficult to obtain complete pathophysiology of diabetic metabolism because glucose and insulin have been influenced through various complex process. However, some metabolic indices are appropriate for investigate clinical diabetic situation, which are HOMA-R, HOMA-β and IGI. Regarding these biomarkers, the standard values widely used so far are HOMA-R<2.5, HOMA-β><2.5, HOMA-β>30%, and IGI>0.4-0.5.
The standard range of HOMA-R cannot be clearly defined, where 2.5 has been often used in usual situation. The cutoff point of HOMA-R was studied in reference to the definition of IDF and Adult Treatment Panel III (ATPIII) criteria.28 The method included the analysis of area under the curve (AUC). As a result, the optimal cut-off of HOMA-R was 1.775 for diagnosis of Metabolic syndrome (MetS) in non-DM patients, 4.325 and 3.875 for diagnosis of DM in MetS patients for IDF and ATPIII, respectively. Consequently, MetS or diabetes can be diagnosed from various patients with or without diabetes in applying this optimal method. HOMA-β has been one of the useful static assessments of β-cell function.29 It is simple and widely applied in epidemiologic investigation. However, it has some limitation because only one point of glucose and insulin cannot represent complex situation of glucose-insulin feedback axis.30
Regarding IGI, lower results were found in case 1 and 4, and normal in case 2 (Table 2). Case 1 has weight reduction compared to 19 months ago, leading to decreased fasting IRI and HOMA-R. However, the capacity of insulin secretion did not change. IGI has been widely used to quantify the response of β-cell for the changes in blood glucose. It is calculated for deltaIRI (0-30min) (µIU/mL)/delta-glucose (0-30-minutes) (mg/dL).24 It is also applied in the case of MTT trials.31 Compared IGI for GTT and MTT has been studied.32 Authors have studied insulin and C-peptide immunoreactivity (CPR) responses for MTT and presented clinical efficacy of MTT.19,21 From several reports, β-cell function seems to be higher during MTT than OGTT, suggesting that several function parameters would be influenced by some composition in the meal.24
Glucagon stimulation test (GST) has been applied for the investigation of insulin-secreting ability from β-cell in the pancreas. Formerly, the purpose was evaluation of residual β-cell function,33 in which subjects were 17 insulin-dependent diabetes mellitus (IDDM) patients. C-peptide elevation was measured after meal (p<0.01) and after intravenous glucagon (p><0.001). Both responses showed correlation (p><0.005), suggesting possible prediction of glucagon test for the responsive ability of β-cells during usual daily life. In the current case, GST revealed that pancreatic β-cell function was preserved (Figure 3). Based on his data for clinical course, 75 g OGTT, HOMA-R, HOMA-β, IGI and glucagon tests were comprehensively analyzed totally. LCD continuation had successfully brought weight reduction, decreased HbA1c, moderately reduced insulin resistance. Furthermore, it seems to maintain secretory capacity against strong endocrinological stimulation. On the other hand, OGTT seems to be rather weaker stimulation compared to GST. IGI values by 75 g OGTT did not improve.
There are some limitations for this report as follows: i) super LCD was continued, but any food has carbohydrate to some degree for elevating blood glucose, ii) correlation between carbohydrate amount and post-prandial glucose was found, but the study of MMT cannot predict complete suggested glucose values due to ingestion the meal per OS, iii) OGTT twice showed delayed IRI responses, which may suppose persisting impaired insulin secretion and increased insulin resistance, iv) current glucose-insulin situation will be possible changed due to clinical course in the future, then detail follow-up the glucose variability would be required.
In summary, this case report described a male patient with T2DM and gave some discussion from several points of view. He successfully continued daily check of meal with carbohydrate amount and post-prandial blood glucose. Diabetic biomarkers including 75 g OGTT, HOMA-R, HOMA-β, IGI and GST were analyzed for evaluating current pathophysiology. Current information will become hopefully some reference for diabetic practice and research in the future.
ETHICAL CONSIDERATIONS
Current investigation was conducted along the Declaration of Helsinki, that was previously revised in 2013 for the WMA Fortaleza General Assembly. In addition, several commentaries were added by the ethical guidelines for medical research. They are notified by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and Ministry of Health, Labour and Welfare (MHLW), Japan. This study in detail was explained to the patient. Authors have obtained the written document agreements from the patient. Current study was discussed in the professional ethical committee. The committee involves several professionals including president, director, doctors, nurses, pharmacists, dieticians, and a professional legal specialty.
FUNDING
There was no funding received for this paper.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.












