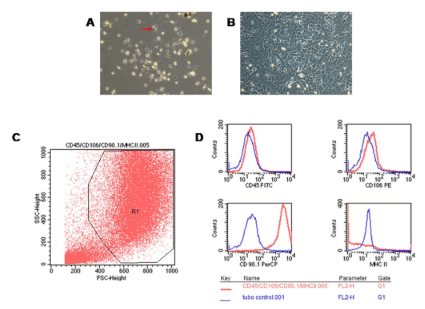
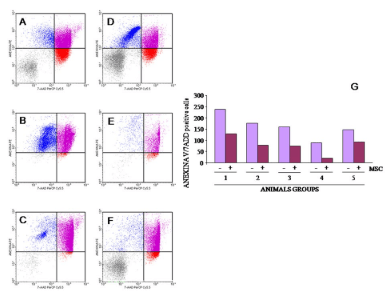
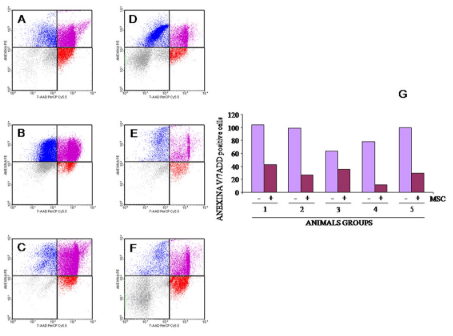
1. Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002; 68(5): 501-510. doi: 10.1002/jnr.10240
2. McDonald JW, Howard MJ. Repairing the damaged spinal cord: A summary of our early success with embryonic stem cell transplantation and remyelination. Prog Brain Res. 2002; 137: 299-309. doi: 10.1016/S0079-6123(02)37023-7
3. Campos LS. Neurospheres: Insights into neural stem cell biology. J Neurosci Res. 2004; 78: 761-769. doi: 10.1002/jnr.20333
4. Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat Biotech. 2001; 19: 1134-1140. doi: 10.1002/jnr.20333
5. Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005; 128(3): 552-563. doi: 10.1053/j.gastro.2004.11.051
6. Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma. 2002; 19(12): 1609-1617. doi: 10.1089/089771502762300265
7. Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002; 22: 6623-6630. doi: 10.1523%2FJNEUROSCI.22-15-06623.2002
8. Pluchino S, Quattrini A, Brambilla E, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003; 422: 688-694. doi: 10.1038/nature01552
9. Pluchino S, Zanotti L, Rossi B, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005; 436: 266-271. doi: 10.1038/nature03889
10. Anderson P, González-Rey E, O’Valle F, Martín F, Oliver FJ, Delagado M. Allogeneic adipose-derived mesenchymal cells ameliorate experimental autoimmune encephalomyelitis by regulating self-reactive T cell reponses and dendritic cell function. Stem Cells Int. 2017. doi: 10.1155/2017/2389753
11. Marín-Bañasco C, Benabdellah K, Melero-Jerez C, et al. Gene therapy with mesenchymal stem cells expressing IFN-β ameliorates neuroinflammation in experimental models of multiple sclerosis. Br J Pharmacol. 2017; 174: 238-253. doi: 10.1111/bph.13674
12. Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, et al. Evaluation of AD-MSC (adipose-derived mesenchymal stem cells) as a vehicle for IFN-β delivery in experimental autoimmune encephalomyelitis. Clin Immunol. 2016; 169: 98-106. doi: 10.1016/j.clim.2016.06.015
13. Yousefi F, Ebtekar M, Soudi S, Soleimani M, Hashemi SM. In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis. Immunol Lett. 2016; 172: 94-105. doi: 10.1016/j.imlet.2016.02.016
14. Shalaby SM, Sabbah NA, Saber T, Abdel Hamid RA. Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model. IUBMB Life. 2016; 68: 106-115. doi: 10.1002/iub.1469
15. Jiang H, Zhang Y, Tian K, Wang B, Han S. Amelioration of experimental autoimmune encephalomyelitis through transplantation of placenta-derived mesenchymal stem cells. Sci Rep. 2017. doi: 10.1038/srep41837
16. Selim AO, Selim SA, Shalaby SM, Mosaad H, Saber T. Neuroprotective effects of placenta-derived mesenchymal stem cells in a rat model of experimental autoimmune encephalomyelitis. Cytotherapy. 2016; 18: 1100-1013. doi: 10.1016/j.jcyt.2016.06.002
17. Torkaman M, Ghollasi M, Mohammadnia-Afrouzi M, Salimi A, Amari A. The effect of transplanted human Wharton’s jelly mesenchymal stem cells treated with IFN-γ on experimental autoimmune encephalomyelitis. Cell Immunol. 2017; 311: 1-12. doi: 10.1016/j.cellimm.2016.09.012
18. Bravo B, Gallego MI, Flores AI, et al. Restrained Th17 response and myeloid cell infiltration into the central nervous sytem by human decidua-derived mesenchymal stem cells during experimental autoimmune encaphalomyelitis. Stem Cell Res Ther. 2016; 7: 43. doi: 10.1186/s13287-016-0304-5
19. Rafieemehr H, Kheyrandish M, Soleimani M. Neuroprotective effects of transplanted stromal cells-derived human umbilical cord blood neural progenitor cells in EAE. Iran J Allergy Asthma Immunol. 2015; 14(6): 596-604.
20. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997; 276: 71-74. doi: 10.1126/science.276.5309.71
21. Bossolasco P, Cova L, Calzarossa C, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005; 193: 312-325. doi: 10.1016/j.expneurol.2004.12.013
22. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S. Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006; 6: 435-441. doi: 10.1016/j.coph.2006.02.008
23. Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009; 113: 6576-6583. doi: 10.1182/blood-2009-02-203943
24. Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002; 1: 92-100. doi: 10.1016/s1474-4422(02)00040-6
25. Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005; 195: 16-26. doi: 10.1016/S1474-4422(02)00040-6
26. Bartholomew A, Polchert D, Szilagyi E, Douglas GW, Kenyon N. Mesenchymal stem cells in the induction of transplantation tolerance. Transplantation. 2009; 87: S55-S57. doi: 10.1097/TP.0b013e3181a287e6
27. Liao W, Pham V, Liu L, et al. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmuneencephalomyelitis. Biomaterials. 2016; 77: 87-97. doi: 10.1016/j.biomaterials.2015.11.005
28. Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. Human mesenchymal stem cells infiltrate the spinal cord, reducede myelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2010; 69: 1087-1095. doi: 10.1097/NEN.0b013e3181f97392
29. Hou Y, Heon Ryu C, Jun JA, Kim SM, Jeong CH, Jeun SS. Interferon β-secreting mesenchymal stem cells combined with minocycline attenuate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014; 274: 20-27. doi: 10.1016/j.jneuroim.2014.06.001
30. Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007; 61: 219-227. doi: 10.1016/j.jneuroim.2014.06.001
31. Kassis I, Grigoriadis N, Gowda-Kurkalli B, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008; 65: 753-761. doi: 10.1001/archneur.65.6.753
32. Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009; 57: 1192-1203. doi: 10.1002/glia.20841
33. Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009; 182: 5994-6002. doi: 10.4049/jimmunol.0803962
34. Liu XJ, Zhang JF, Sun B, et al. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-beta and interleukin-6. Clini Exp Immunol. 2009; 158: 37-44. doi: 10.1111/j.1365-2249.2009.03995.x
35. Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia. 2001; 36: 220-234. doi: 10.1002/glia.1111
36. Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999; 29: 3432-3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2
37. McLaughlin KA, Wucherpfennig KW. B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv Immunol. 2008; 98: 121-149. doi: 10.1016/S0065-2776(08)00404-5
38. Shoham T, Parameswaran R, Shav-Tal Y, Barda-Saad M, Zipori D. The mesenchymal stroma negatively regulates B cell lymphopoiesis through the expression of activin A. Ann NY Acad Sci. 2003; 996: 245-260. doi: 10.1111/j.1749-6632.2003.tb03253.x
39. Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006; 24: 386-398. doi: 10.1634/stemcells.2005-0008
40. Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003; 195: 72-80. doi: 10.1034/j.1600-065X.2003.00063.x
41. Amé-Thomas P, Maby-El HH, Monvoisin C, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: Role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007; 109: 693-702. doi: 10.1182/blood-2006-05-020800
42. De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003; 89: 267-270. doi: 10.1016/S0165-2478(03)00108-1
43. Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106: 1755-1761. doi: 10.1182/blood-2005-04-1496
44. Yu JW, Li YN, Song GB, et al. Synergistic and superimposed effect of bone marrow-derived mesenchymal stem cells combined with fasudil in experimental autoimmune encephalomyelitis. J Mol Neurosci. 2016; 60: 486-497. doi: 10.1007/s12031-016-0819-3
45. Togha M, Jahanshahi M, Alizadeh L, et al. Rapamycin augments immunomodulatory properties of bone marrow-derived mesenchymal stem cells in experimental autoimmune encephalomyelitis. Mol Neurobiol. 2017; 54: 2445-2457. doi: 10.1007/s12035-016-9840-3
46. Wang D, Li SP, Fu JS, Bai L, Guo L. Resveratrol augments therapeutic efficiency of mouse bone marrow mesenchymal stem cell-based therapy in experimental autoimmune encephalomyelitis. Int J Dev Neurosci. 2016. doi: 10.1016/j.ijdevneu.2016.01.005
47. Hao F, Li A, Yu H, et al. Enhanced neuroprotective effects of combination therapy with bone marrow-derived mesenchymal stem cells and Ginkgo biloba extract (EGb761) in a rat model of experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 2016; 23: 41-57. doi: 10.1159/000437429
48. Luz-Crawford P, Kurte M, Bravo-Alegría J, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013; 4: 65. doi: 10.1186/scrt216
49. Weissert R, Wallstrom E, Storch MK, et al. MHC haplotype-dependent regulation of MOG-induced EAE in rats. J Clin Invest. 1998; 102: 1265-1273. doi: 10.1186/scrt216
50. Villarón EM, Almeida J, López-Holgado N, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004; 89(12): 1421-1427.
51. Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315-317.
52. Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005; 305: 33-41. doi: 10.1016/j.yexcr.2004.12.013
53. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 105: 2821-2827. doi: 10.1182/blood-2004-09-3696
54. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105: 1815-1822. doi: 10.1182/blood-2004-04-1559
55. Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005; 105: 4120-4126. doi: 10.1182/blood-2004-02-0586
56. Le BK, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scan J Immunol. 2003; 57: 11-20. doi: 10.1046/j.1365-3083.2003.01176.x
57. Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003; 171: 3426-3434. doi: 10.4049/jimmunol.171.7.3426
58. Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99: 3838-3843. doi: 10.1182/blood.V99.10.3838
59. Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004; 103: 4619-4621. doi: 10.1182/blood-2003-11-3909
60. Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007; 25: 1753-1760. doi: 10.1634/stemcells.2007-0068
61. Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107: 367-372. doi: 10.1182/blood-2005-07-2657
62. Traggiai E, Volpi S, Schena F, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008; 26: 562-569. doi: 10.1634/stemcells.2007-0528
63. Rasmusson I, Le BK, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scan J Immunol. 2007; 65: 336-343. doi: 10.1111/j.1365-3083.2007.01905.x
64. Tabera S, Pérez-Simón JA, Díez-Campelo M, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008; 93: 1301-1309. doi: 10.3324/haematol.12857
65. Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003; 101: 3722-3729. doi: 10.1182/blood-2002-07-2104
66. Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002; 61: 1013-1021. doi: 10.1093/jnen/61.11.1013
67. Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal BA, Khademi M. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol. 2008; 84(5): 1248-1255. doi: 10.1189/jlb.1207844
68. Jack CS, Arbour N, Blain M, Meier UC, Prat A, Antel JP. Th1 polarization of CD4+ T cells by Toll-like receptor 3-activated human microglia. J Neuropathol Exp Neurol. 2007; 66: 848-859. doi: 10.1097/nen.0b013e3181492a7
69. Williams KC, Ulvestad E, Hickey WF. Immunology of multiple sclerosis. J Clin Neurosci. 1994; 2: 229-245.
70. Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. New Eng J Med. 2008; 358: 676-688. doi: 10.1056/NEJMoa0706383
71. Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007; 178: 6092-6099. doi: 10.4049/jimmunol.178.10.6092
72. Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: Application to autoimmune disease therapy. Cell. 1995; 80(5): 707-718. doi: 10.1016/0092-8674(95)90349-6