INTRODUCTION
Various eosinophilic disorders have been described in horses and Multisystemic Eosinophilic Epitheliotropic Disease (MEED), also known as Hypereosinophilic syndrome (HES) due to the abundant eosinophils infiltrating many organs, although rare, is the most reported.1,2 Other disorders characterized by prevalent eosinophilic infiltrates affecting specific organ systems, as small and large intestine, skin and lung have been rarely described.3-7 Eosinophilic infiltrates of equine lungs are observed during parasitic infections or allergic condition8,9 but eosinophilic pneumonia with vasculitis, which are not associated with parasitic infection and without the evidence of involvement of other organs have not been previously reported in horses. In the present study, we describe the gross and microscopic lesions of idiopathic eosinophilic pneumonia with associated vasculitis in 11 horses.
MATERIALS AND METHODS
During a 3-years period, lungs from 125 horses with gross signs of pulmonary disease were collected at different slaughterhouses in Italy. Representative tissues were fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin. Tissue sections of 4 µm were stained with Hematoxylin and Eosin (HE), Giemsa, Gram, Periodic Acid-Schiff (PAS), Grocott’s Methenamine Silver (GMS), toluidine blue, Ziehl-Neelsen acid-fast and examined microscopically.
RESULTS
In 11 horses (ages ranged from 4 to 18 years; mean 9 years), lungs were moderately enlarged, pale pink, poorly collapsed and showed variable to high numbers of randomly distributed nodule switch were 0.4-4.0 cm-diameter, firm and white-reddish in color (Figures 1A and 1B). The pulmonary lymph nodes were mildly to moderately enlarge. At gross exam no macroscopic lesion were seen in small and large intestine, liver, spleen and kidney. Histologically lung lesions ranged from mild to moderate eosinophilic bronchointerstitial pneumonia (Figure 2A), to severe eosinophilic lobular bronchopneumonia with edema and fibrinous exudate (Figure 2B). In the bronchointerstitial pattern the bronchioles appeared mildly to moderately hyperplastic, with variable numbers of intraepithelial eosinophils (Figure 3A), often the underlying lamina propria appeared moderately expanded by numerous eosinophils and low number of lymphocytes (Figure 3B), sometimes the Bronchus-associated lymphoid tissue (BALT) were hyperplastic (Figure 3C). In the lobular pattern of pneumonia, large number of eosinophils filled the alveolar spaces with admixed few lymphocytes, sloughed necrotic cells and fibrin (Figure 3D). In this pattern, frequently the interlobular septa and the pleura appeared expanded by fibrin, edema and many eosinophils (Figures 3E and 3F). The other part of parenchyma was occasionally emphysematous with mild interstitial fibrosis. In all this cases, within the inflammatory foci as well as at the periphery, small to medium-size vessels had degenerative and necrotic changes with concomitant eosinophilic inflammation. The small arteries appeared surrounded by a densely cellular eosinophilic infiltrate, with admixed numerous erythrocytes (hemorrhages), while the vascular wall had transmural infiltration of eosinophils and severe vacuolar degeneration of smooth muscle cells of tunica media (Figures 4A and 4B). The arterioles appeared thickened, distorted and surrounded by a severe perivascular eosinophilic infiltrate together with edema and hemorrhages, also the wall appeared infiltrated and disrupted by eosinophils, together with deposition of fibrin and presence of karyorrhectic debris and pyknotic endothelial cells (fibrinoid necrosis) (Figures 4C, 4D, 4E and 4H). There was no histologic evidence of parasites or inorganic material within the lesion of any lung tissues examined. Histochemical stains for fungi (PAS and GMS), bacteria (Gram, Giemsa) and acid-fast bacteria (Ziehl-Neelsen) were negative.
Figure 1: Lung multifocal white-grey to red nodules randomly distributed. (A) Few small nodules (arrow); (B) Numerous nodules; (C) Large nodules; (D) Cut section; (E) Hemorrhagic appearance on cut section.
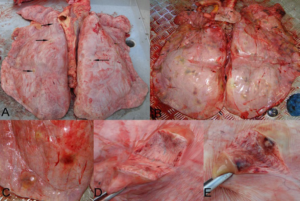
Figure 2: (A) Mild eosinophilic bronchointerstitial pneumonia. (B) Severe eosinophilic lobular bronchopneumonia with edema (HE, A & B: 20X).
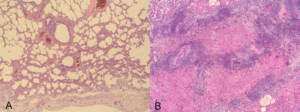
Figure 3: Different microscopic features of eosinophilic pneumonia: eosinophilic bronchiolitis and peribronchiolitis. (A, B) BALT hyperplasia. (C) Eosinophilic alveolitis. (D) Interlobular fibrinous exudate. (E) Eosinophilic pleuritis. (F) (HE, A: 20X; B-C-D-E-F: 10X).
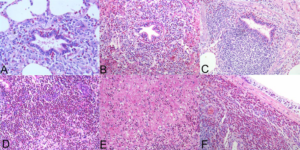
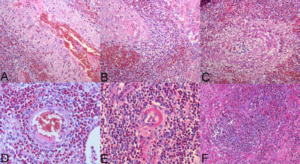
Figure 4: Various features indicating vasculitis: perivascular eosinophilic infiltrate, hemorrhages, transmural vasculitis, edema, disruption, (A-B-C-D) Fibrinoid necrosis of the vascular wall. (E) Necrosis and inflammation. (F) (HE, A-B-C-F: 10X; D-E: 20X).
DISCUSSION
In horse, pulmonary disorders associated with eosinophilic infiltrate include: MEED, parasitic infection, allergic condition during Recurrent Airway Obstruction (RAO) or idiopathic eosinophilic pneumonia (Table 1).1,2,7,8 MEED is histologically characterized by eosinophilic and lymphoplasmacytic infiltrates and the formation of typical eosinophilic granulomas in multiple organs.1,2 Granulomas are most commonly found in the gastrointestinal tract and skin, but also in pancreas, salivary glands and lungs. Histologic findings observed in our case series do not resemble the eosinophilic pulmonary granulomas generally described in horses with MEED. Moreover prominent vasculitis involving small to medium size vessels, as detected here, is a pathological finding never described in horses with MEED.
Table 1: Pulmonary disorders of horse with associated tissue eosinophilia.
| MEED (Multisystemic eosinophilic epitheliotropic disease) or hypereosinophilic syndrome |
| RAO (respiratory airway obstruction)-hypersensitivity reaction |
| Parasitic bronchopneumonia (Parascarisequorumm, Dictyocaulusarnfieldi) |
| Idiopathic eosinophilic pneumonia |
Bronchiolitis with associated variable amount of eosinophils is an occasional feature of RAO or heaves in adult horses, but the severe infiltration of eosinophils within alveoli associated with vasculitis, detected in these cases, are not a finding of RAO.9 In RAO the most typical histological findings involve small bronchioles as accumulation of mucus and neutrophils in lumina, thickening of the bronchiolar wall by goblet cells hyperplasia and infiltration of lamina propria by lymphocytes, plasma cells, mast cells and occasionally eosinophils.9
Nodular pulmonary lesions with associated tissue eosinophilia may be caused by larval migration of Parascarisequorum in young horses.9 Also, Dictyocaulus arnfieldi is occasional ly responsible for eosinophilic bronchiolitis in horse and only in young foals the infection is patent.8,9 Larval migration of Strongylus vulgar is has been associated with eosinophilic pneumonia in experimentally infected foals but spontaneous pulmonary diseases caused by S. vulguaris or Strongylus edentatus infections are considered unusual and caused by aberrant migration.10
In our cases, serial sections did not ever allow identifying remnants of adult worms, or larvae or eggs neither foci of mineralization within the lesions and the older ages of the horses together with the fact that no other organs had lesions related to parasitic infection make a parasitic aetiology unlikely.
Bell et al11 reported clinical signs, clinicopathological data, radiographic and histopathological findings in 7 horses with eosinophilic pneumonia of unknown etiology. The authors highlighted the similarity between their cases and Idiopathic Chronic Eosinophilic Pneumonia (ICEP) in humans. The histophatological findings were consistent with interstitial eosinophilic infiltrates in lungs but detailed descriptions of the histological lesions were available only for two horses included in the study.7 Also, Uhlhorn et al6 described a case of idiopathic eosinophilic pneumonia in a pony characterized by multiple eosinophilic granulomas. In both studies, vasculitis was not reported.6,7
In the present case series based on histologic findings and the absence of an etiologic agent, a diagnosis of idiopathic eosinophilic pneumonia with associated vasculitis was made. Interestingly vasculitis has not been described in lungs of horse with eosinophilic pneumonia before this study.
In human medicine eosinophilic pneumonia with associated necrotizing vasculitis affecting small to medium-sized vessels and extra vascular granulomas are classically described findings in Chrug-Strauss Syndrome (CSS), also known as Eosinophilic granulomatosis with polyangiitis (EGPA).12,13,14 This syndrome differs from other small-vessel vasculitis by the presence of severe asthma and blood and tissue eosinophilia. CSSEGPA is considered an idiopathic condition and various authors suggest that a T-helper-2 and B-cell immune responses with up-regulation of Interleukin-4 (IL-4), Interleukin-5 (IL-5) and Interleukin 13 (IL-13) and subsequent differentiation and activation of eosinophil play a major role in its pathogenesis.15,16,17 The vasculitis observed in the horses in this case series share many similarities with that described in CSS-EGPA, namely, the size of vessel involved and the pattern of inflammation with fibrinoid necrosis.
CONCLUSION
In veterinary medicine, eosinophilic disease in horse primarily affecting the pulmonary system and not associated with parasitic infection or MEED is rarely described. The etiology of the lesions observed in these cases remains undetermined, but based on the findings described here, especially the vasculitis with fibrinoid necrosis, an immune-mediated reaction directed at target antigens of various origins (for example drugs, pathogens or vaccines) along with high levels of IL-4, IL-5 and IL-13 leading to a Chronic Eosinophilic Pneumonia (CEP) and severe vasculitis seems a likely explanation.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL CONSIDERATION
Hereby, Fulvio Laus, The President of Animal-welfare body has been certified that in authors institution, the University of Camerino, has been instituted and is operative the “Animal-welfare body” as required by the European directive 2010/63/eu on the protection of animals used for scientific purpose.









