INTRODUCTION
Significant advances in space travel have resulted in the Perseverance Rover landing on the planet Mars (February 18, 2021) with manned space flight planned for 2026.1 However, the question remains as to how to best equip the astronauts for dealing with prolonged stays in microgravity environments. Exposure to the weightlessness experienced in microgravity environments leads to bone loss,2 muscle atrophy3 and cardiovascular deconditioning.4 Accelerated bone loss in crew members in space is considered a critical risk factor for the early onset of osteoporosis after return to earth.5 Astronauts also experience deconditioning of the cardiovascular system that increases with the duration of microgravity exposure. The limited capabilities of research during space flight, due to time and costs, have led to ground-based models that simulate microgravity.6 In humans, the ground-based model is bed rest with head-down tilt and results in similar physiological adaptations to those seen during spaceflight.7 These include reduced blood volume,8 increased venous compliance,9 reduced maximal aerobic capacity,10 and impaired carotid baroreflex responses7 which can contribute to orthostatic intolerance.
Orthostatic intolerance reflects a decrement in one or more of the mechanisms responsible for blood pressure adaptations to changing posture. Various mechanisms that have been proposed as contributing to this condition include hypovolemia,8 an attenuated reflex activation of sympathetic nervous system activity,11 and decreases in vasoconstrictive responses.12 The functional changes in the vascular component of the baroreceptor reflex appear due to attenuation of the normal vasoconstriction that occurs with standing.12,13 Ground-based animal models, such as hindlimb suspended (HLS) in the rat, have also demonstrated a decrease in plasma volume,14 diminished baroreflex function15 and indications of orthostatic intolerance.14
Vasoconstriction is thought to be the primary response to hyperoxia and has been observed in animals, healthy volunteers16 and patients.17 Therefore, we proposed that the attenuated vasoconstrictive response due to 28-days of HLS would be offset by hyperbaric oxygen treatment (HBO) treatments.
METHODS
Animals
All procedures performed in this study were approved by the Institutional Animal Care and Use Committee of The University of Texas at Arlington.
Forty male Sprague-Dawley rats weighing approximately 350 g were obtained (Harlan) and housed in a temperature controlled (23±2 °C) room with a 12:12 hour light-dark cycle. Rats were divided into four groups: Aging controls (AC, n=10), hindlimb suspension (HLS, n=10), aging controls treated with hyperbaric oxygen (AC-HBO, n=10), and hindlimb suspended treated with hyperbaric oxygen (HLS-HBO, n=10). Water and rat chow were provided, but while the HLS animals were free fed, the control groups were fed the previous day’s average consumption of the experimental animals.
One week after arrival, rats were randomly assigned to control or HLS groups. HLS animals were placed in a head-down position of about 30° from horizontal using a harness attached to the tail. The harness was made of two narrow strips of cloth tape pressed together, folded in half with a hooked anchored at the fold. Marine Goop (Eclectic Products, Inc., Pineville, LA, USA) was applied along the inside of the cloth tape and then adhered along the lateral sides of the tail at approximately the middle 3/5 of the tail. The hook in the harness was attached to a swivel suspended from a wire stretched across the top of the cage. The height of the hindlimb elevation was adjusted to prevent the hindlimbs from touching the floor of the cage. The forelimbs remained in contact with the floor of the cage allowing movement around the entire cage. Hindlimb unloading was maintained for 4-weeks.
Hyperbaric Oxygen Treatment
One of the HLS groups and one of the control groups underwent HBO therapy six days a week for four-weeks. The treatment was 1.5-hours in duration at a depth of 2.5 atmospheres absolute (ATA; 22.5 psi) with 100% oxygen. Rats were placed in a cage made of acrylic with aluminum bracing which allowed for continual HLS. The cage was designed to fit in the animal hyperbaric chamber (Gulf Coast Hyperbarics, Panama City, FL, USA) and accommodated six rats (3 HLS and 3 non-HLS). Acrylic dividers kept the rats separated.
The chamber was flushed for 30-seconds to allow room air to be replaced with 100% O2, then pressurized at a rate of 2.5 psi/min to the predetermined depth using 100% oxygen. During the treatment, oxygen was vented at a rate of approximately 5-7 L/min to prevent carbon dioxide buildup in the chamber but still allowing the pressure level to be maintained. Decompression rate was also 2.5 psi/min until sea level was reached, at which time the rats were removed from the chamber and returned to their cages. Compression (pressurization) and decompression each added 10 additional minutes to the treatment, thus the rats were in the chamber for a total of 110-minutes.
Vessel Preparation
At the end of the 28-days of hindlimb unweighting, the rats were sedated using Isoflourane (1.0-1.5%). While sedated, the femoral arteries were isolated. The thoracic cavity was opened, exposing the heart which was perfused externally with cold saline. Sections were removed from the carotid and thoracic aorta vessels.
Under a dissecting microscope the segments were trimmed of fat and connective tissue and cut into 1-2 mm lengths and mounted on two stainless steel wires that were passed through the vessel lumen. One wire was attached to a fixed end and the other to a force transducer so that the vessels could be stretched to produce 0.5 g of tension on the femoral artery, 1.0 g on the carotid artery, and 1.5 g on the aorta. The resting tensions of the vessels were allowed to stabilize for 30-60-minutes before pre-constriction with phenylephrine (PHE). The vessels suspended in a modified Krebs-Henseleit tissue bath of the following composition (mM): NaCl 115, NaCO3 20, KCl 4.0, K2 HPO4 0.9, MgSO2 1.1, and glucose (C6 H12O6 ) bubbled with 95% O2 and 5% CO2 to achieve a pH of 7.4 and temperature was maintained at 37 °C. Once the vessel tension was stabilized, they were submaximally pre-constricted in a final PHE concentration of 3×10-7 M. After pre-constriction, relaxation in response to cumulative doses of acetylcholine (Ach; 10-10-10-4 mol/L for the aorta and 10-9-10-4 mol/L for the carotid and femoral arteries) and sodium nitroprusside (SNP; 10-10-10-4 mol/L for all arteries) were measured. Relaxation responses were expressed as a percentage of the PHE induced pre-constriction. Constriction of arterial rings was also determined in response to increasing concentrations to PHE (10-10-10-4 mol/L). Constriction response was expressed as a percentage of resting vessel tension. Data was collected on Power Lab equipment (ADInstruments, Colorado Springs, CO, USA) using Chart 5 Software (ADInstruments, Colorado Springs, CO, USA) and concentration-response curves were produced using Table Curve software (ADInstruments, Colorado Springs, CO, USA).
Statistical Analyses
For ACh, SNP and PHE, data from each animal were fit to a sigmoidal function, using a four-parameter (minimum, maximum, EC50, and slope) logistic equation (Table Curve 2D 25.0, SPSS Inc., Chicago, IL, USA). A 2×2 analysis of variance with interaction was used to determine effects of HBO and HLS treatments on vessel relaxation. Main effects were HBO (treated vs. non-treated) and HLS status (treated vs. non-treated). Thus, an effect of HBO on the response indicates that the combined HBO-treated group (AC-HBO+HLS-HBO) is different from the combined non-HBO-treated group (AC+HLS). Similarly, an effect of HLS on the response indicates that the combined HLS-treated group (HLS+HLS-HBO) is different from the combined non-HLS-treated group (AC+AC-HBO). Because L-NAME data did not fit a sigmoidal function, they were analyzed using a repeated measures analysis of variance testing for HLS and HBO main effects. Significance was accepted when p≤0.05.
RESULTS
Aortae
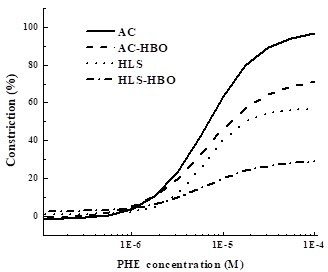
Phenylephrine: 2×2 ANOVA results revealed significant effects of both HLS and HBO in the maximal contractile response of aortae to PHE (Table 1; Figure 1). HLS-treated rats had a significantly lower maximum contractile response to PHE compared with non HLS treated rats (p=0.0001). HBO-treated rats also had significantly lower maximum contractile responses to PHE compared with non-HBO-treated rats (p=0.01). Further, HLS-treated groups had a higher minimum contractile response to PHE (p=0.01). There were no effects of HLS or HBO on EC50 or slope parameters for the aorta PHE analysis.
Figure 1. Dose-Response Relationship between Phenylephrine (PHE) and % Constriction in Rat Aortae
| Table 1. Responses of Aortae to Phenylephrine in Hindlimb-unweighted and Hyperbaric Oxygen-Treated Rats |
|
AC |
AC-HBO |
HLS |
HLS-HBO |
| Phenylephrine |
| Minimum (%) |
-1.83±0.87 |
0.97±0.67 |
0.96±0.66§ |
2.50±2.02§ |
| Maximum (%) |
98.2±9.5 |
73.0±8.2* |
57.0±13.3§ |
9.4±3.8* § |
| EC50 (log M) |
-6.6±0.1 |
-6.5±0.04 |
-6.5±0.1 |
-6.4±0.1 |
| Slope |
1.51±0.10 |
1.34±0.06 |
2.05±0.56 |
1.44±0.16 |
Data expressed as Mean±SE.
AC= Aging control;
HBO=Hyperbaric oxygen-treated;
HLS=Hindlimb suspended.
*p≤0.05, combined HBO-treated group < combined non-HBO-treated group;
§ p≤0.05, combined HLS-treated group different from combined non-HLS-treated group. |
Acetylcholine: 2×2 ANOVA results indicated that HLS-treated groups had a lower slope of the ACh dose-relaxation response curve (p=0.03; Table 2). There was a higher maximum relaxation response in HBO-treated groups compared with non-HBO-treated groups (p=0.06), however, this was not significantly different. In response to ACh in the presence of L-NAME (Table 3), HLStreated groups exhibited significantly less inhibition (i.e, there was greater relaxation, p=0.0001). Follow-up analyses indicated that this effect was significant at all doses of ACh (all p≤0.001). However, the HBO effect for ACh relaxation in the presence of LNAME (p=0.07) was not significant.
| Table 2. Responses of Aortae to Acetylcholine and Nitroprusside in Hindlimb-Suspended and Hyperbaric Oxygen-Treated Rats |
|
AC |
AC-HBO |
HLS |
HLS-HBO |
| Acetylcholine |
| Minimum (%) |
0.87±0.60 |
3.23±1.16 |
2.07±1.14 |
0.16±2.35 |
| Maximum (%) |
74.3±5.3 |
94.0±4.1 |
78.9±4.4 |
88.9±5.58 |
| EC50 (log M) |
-6.66±0.08 |
-6.74±0.05 |
-6.67±0.07 |
-6.74±0.16 |
| Slope |
0.95±0.06 |
0.84±0.05 |
0.77±0.03§ |
0.77±0.06§ |
| Nitroprusside |
| Minimum (%) |
0.20±0.60 |
0.47±0.27 |
4.85±2.63* |
-0.87±0.95 |
| Maximum (%) |
121.6±5.3 |
114.3± 4.1 |
114.0±7.4 |
117.3±6.6 |
| EC50 (log M) |
-7.49±0.28 |
-6.78±0.98 |
-7.93±0.07 |
-7.34±0.14 |
| Slope |
0.86±0.04 |
0.80±0.03 |
0.76±0.05§ |
0.74±0.03§ |
Data expressed as Mean±SE.
AC=Aging control;
HBO=Hyperbaric oxygen-treated;
HLS=Hindlimb suspended. *p≤0.05, greater than other groups;
§ p≤0.05, combined HLS-treated group different from combined non-HLS groups. |
| Table 3. Relaxation Responses of Aortae to Acetylcholine in the Presence of L-NAME in hindlimb-Suspended and Hyperbaric Oxygen-treated Rats |
|
AC (n=8) |
AC-HBO (n=9) |
HLS (n=7) |
HLS-HBO (n=5) |
| Relaxation (%) |
| 10-10 M |
-2.70±0.89 |
0.28±0.65 |
3.86±1.40* |
6.56±3.18* |
| 10-9 M |
-3.23±1.18 |
0.78±0.94 |
6.83±2.41* |
7.10±3.39* |
| 10-8 M |
-4.13±1.10 |
0.82±1.33 |
7.96±2.89* |
7.93±2.50* |
| 10-7 M |
-5.17±1.07 1 |
1.33±1.48 |
7.97±3.05* |
7.99±3.54* |
| 10-6 M |
5.57±1.01 |
1.67±1.76 |
7.84±3.17* |
8.48±3.24* |
| 10-5 M |
-6.68±0.98 |
0.96±1.95 |
7.43±2.89* |
6.91±3.74* |
| 10-4 M |
9.95±2.18 |
0.34±2.28 |
7.18±2.66* |
5.61±3.52* |
Data expressed as Mean±SE.
AC=Aging control;
HBO=Hyperbaric oxygen-treated;
HLS=Hind limb suspended.
*p≤0.05, HLS-treated groups>non-HLS-treated groups. |
Nitroprusside: 2×2 ANOVA revealed that there was an interaction effect of HLS and HBO on the minimum relaxation response (p=0.04), with the HLS group having a greater minimum relaxation (Table 2). There was also an HLS effect on the slope of the SNP dose-relaxation response curve, with HLS-treated groups having a significantly lower slope compared with non-HLS-treated groups (p=0.04).
Carotid Arteries
Acetylcholine
2×2 ANOVA indicated an HLS×HBO interaction for the maximum ACh relaxation (p=0.07, Table 4) but this was not significant. There were no HBO or HLS effects for the minimum, EC50 or slope values. There were also no significant HLS or HBO effects for the response to ACh in the presence of L-NAME (Table 5).
| Table 4. Responses of Carotid Arteries to Acetylcholine and Nitroprusside in Hindlimb-suspended and Hyperbaric Oxygen-treated Rats |
|
AC |
AC-HBO |
HLS |
HLS-HBO |
| Acetylcholine |
| Minimum (%) |
1.04±3.14 |
0.59±3.69 |
6.10±4.90 |
16.58±6.98 |
| Maximum (%) |
76.4±15.8 |
84.0±9.8 |
88.7±13.0 |
101.3±18.6 |
| EC50 (log M) |
-5.70±0.27 |
-6.68±0.56 |
-6.15±0.27 |
-6.13±0.22 |
| Slope |
0.48±0.08 |
0.56±0.14 |
1.10±0.56 |
0.75±0.11 |
| Nitroprusside |
| Minimum (%) |
-36.90±18.00 |
7.07±13.64 |
32.10±34.31 |
-6.30±3.21 |
| Maximum (%) |
130.4±21.3 |
102.1±7.2 |
169.4±77.7 |
110.6±34.5 |
| EC50 (log M) |
-7.88±0.11 |
-6.54±1.50 |
-7.80±0.15 |
-7.36±0.29 |
| Slope |
0.93±0.09 |
2.13±0.77 |
1.86±0.44 |
1.08±0.09 |
Data expressed as Mean±SE.
AC=Aging control;
HBO=Hyperbaric oxygen-treated;
HLS=Hind limb suspended. |
| Table 5. Relaxation Responses of Carotid Arteries to Acetylcholine in the Presence of L-NAME in Hindlimb-suspended and hyperbaric Oxygen-treated Rats |
|
AC |
AC-HBO |
HLS |
HLS-HBO |
| Relaxation (%) |
| 10-9 M |
-11.47±1.86 |
-2.28±11.76 |
-15.01±5.66 |
-25.04±15.60 |
| 10-8 M |
-12.30±2.80 |
-8.27±15.08 |
-6.88±4.82 |
-27.19±18.54 |
| 10-7 M |
-3.71±7.97 |
-10.78±17.19 |
-10.60±5.75 |
-29.32±19.59 |
| 10-6 M |
-5.60±6.25 |
-7.70±15.20 |
-11.32±2.61 |
-29.23±19.48 |
| 10-5 M |
-7.02±5.30 |
-4.13±13.08 |
-16.20±3.83 |
-33.21±20.02 |
| 10-4 M |
-7.93±5.05 |
-11.06±6.11 |
-37.54±14.81 |
-39.74±19.40 |
Data expressed as Mean±SE.
AC=Aging control;
HBO=Hyperbaric oxygen-treated;
HLS=Hind limb suspended.
*p≤0.05, HLS-treated groups>non-HLS-treated groups. |
Nitroprusside
There was an HLS×HBO interaction for the minimum SNP relaxation response (p=0.04; Table 4). There were no HBO or HLS effects for the maximum, EC50 or slope values.
DISCUSSION
The main finding of this study was that the HLS protocol reduced the maximum contractile response of isolated aortic rings to PHE and HBO treatments did not restore contractile function. The HBO treatments further reduced the maximum contractile response to PHE. We hypothesized, based on previous research, that the vasoconstrictive response of the hyperbaric oxygen treatments would offset the lack of vasoconstrictor response seen in those unable to complete a postural challenge following spaceflight.13,16 However, this did not occur, which is seen in Figure 1. Instead, the present findings indicate that the vasodilatory effect becomes pronounced when the oxygen exposure is more extensive.
Orthostatic intolerance has been described as resulting from decreases in one or more mechanisms responsible for the control of blood pressure. These include hypovolemia,8 decreased activation of sympathetic nervous system activity,11 and decreases in vasoconstrictive responses.12 The efforts of this study had been to offset the loss of vasoconstrictive responses, seen with HLS, through intermittent vasoconstriction with HBO. The vasoconstrictive effects of HBO have been attributed to direct effects on vascular smooth muscle,18 alterations in endothelial-derived vasoactive agents such as nitric oxide19 and endothelin-1.20 Hyperbaric oxygen treatments do appear able to modulate vascular responses in various pathological conditions which suggests further study is needed. Vasoconstriction with HBO has been documented with significantly elevated levels of endothelin-1 in patients with peripheral arterial disease. Interestingly, the nitric oxide levels were also significantly elevated, but only in those with a more severe level (Stage 2) of the disease.21
Our purpose had been to determine whether the use of HBO during simulated microgravity might be a useful alternative during spaceflight to offset the occurrence of orthostatic hypotension by maintaining or restoring the vasoconstrictive response. The finding that the combination of HLS and HBO reduced vasoconstrictive capacity suggests that the use of HBO in space would not improve the orthostatic intolerance often experienced following spaceflight. However, the findings that HBO does have a role in modulating vascular responses to vasoconstrictors (phenylephrine) and vasodilators (acetylcholine) may be important in understanding the effects of HBO in pathological conditions of the vasculature.22
An example of the beneficial effects was seen in the improvement of vascular endothelial function in patients undergoing coronary stent implantation. It was suggested that HBO might have the potential to alter the course of coronary artery disease in the future.23 This is in line with our previous findings that HBO causes regression of atherosclerosis in rabbits.24 Another beneficial role for HBO may be in the treatment of diabetes mellitus (DM) as the trend of an increase in the number of patients is continuing.25 Studies using HBO to treat DM have shown improvement in microcirculation,26 angiogenesis,27 and reducing inflammation.28
CONCLUSION
The original purpose of this study resulted in findings that indicate HBO treatments should not be recommended for astronauts during spaceflight and could possibly exacerbate orthostatic intolerance in most astronauts. However, the results here support other findings that HBO may be useful in improving vascular complications associated with pathologic conditions such as DM.29
ACKNOWLEDGEMENTS
We wish to thank Joan F. Carroll, Ph.D. and Patricia A. Gwirtz, Ph.D. for allowing us the use of their laboratories in processing the vessels. Our appreciation goes also to Laurie Massey, M.S., Suzanne Dangelmaier, M.S., and Woineshet Zenebe, Ph.D. for their invaluable assistance in handling the animals.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






