INTRODUCTION
In wild ruminants, sexual maturation concludes in puberty. The end of the prepubertal period marks the beginning of the reproductive capacity. Puberty, which only occurs in animals showing sufficient body development, coincides with the species’ period of mating activity; at this time, photoperiod-induced morphological changes occur in the reproductive tract, spermatogenesis is initiated, and sexual behaviour is first shown. In the Iberian ibex; however, social interactions appear to play an important role in the establishment of puberty, sometimes delaying reproductive behaviour until the age of 2-3 years.1 In humans and domestic animals, puberty can be delayed due to gonadotrophin deficiency, a condition usually present from birth, but this has not been confirmed in wild mountain ruminants. The aim of this case report was to evaluate the efficacy of human chorionic gonadotropin treatment (hCG) to increase the plasma testosterone concentrations in a male Iberian ibex with sexual immaturity.
CASE HISTORY
Iberian ibex sperm samples are routinely collected and cryopreserved at the Animal Reproduction Department of the INIA (Madrid, Spain); the donor animals are kept in captivity as a part of the conservation program. Sperm are collected after anesthetization with 50 µg/kg intravenous detomidine (Domosedan; Pfizer Inc., Amboise Cedex, France), 0.5 mg/kg ketamine hydrochloride (Imalgene 1000; Rhône Mérieux, Lyon, France), and 0.5 mg/kg tiletamine-zolazepam (Zoletil 100; Virbac España S.A., Barcelona, Spain), maintained with 1.5% isoflurane (Isobavet; Intervet/Schering Plough Animal Health, Madrid, Spain) in oxygen (flow rate 2.5 L/min) administered via an endotracheal tube (all animals are placed in the lateral recumbent position). A pulse oximeter is used to monitor the condition of animals throughout. After clipping the hair around the penis and cleaning the surrounding area, the latter organ is manually made to protrude, and maintained protruded with the help of gauze placed caudal to the glans. Semen is obtained by transrectal ultrasound-guided massage of the accessory sex glands (TUMASG) combined with electroejaculation.2 Anaesthesia is then reversed via the administration of 0.7 mg/kg yohimbine hydrochloride (Sigma, St. Louis, MO, USA) (half of the dose injected intravenously, the other half intramuscularly). The animals recover fully within 16 min.
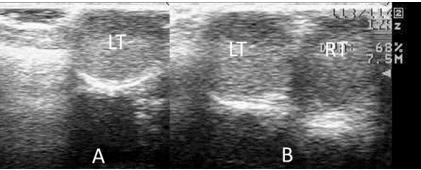
During the rutting season (December), signs of sexual immaturity were detected in a 2.5 year-old-male Iberian ibex, from which sperm could not be collected by the normal procedure. A lack of inguinoscrotal migration of the right testicle was detected by palpation of the testes, and right cryptorchidism was diagnosed. In addition, the urethral process completely adhered to the preputial mucous membrane (Figures 1A and 2A). To complete the examination, the testicular diameter and the echotexture and size of the accessory sex glands were determined by transscrotal and transrectal ultrasonography respectively, employing a 7.5 MHz linear array probe (Prosound 2, Aloka CO., LTD, Tokyo, Japan). The non-descended testicle was smaller than the left testicle (diameter 1.49 cm compared to 1.86 cm), and hypoechogenic. The accessory sex glands were small3 but normal in appearance (bulbourethral gland area 0.25 cm2, mean seminal vesicle area 0.21 cm2).
The ibex was treated with five injections of 500 UI hCG (VeterinCorion®, DivasaFarmavic S.A, Barcelona, Spain) administered intramuscularly for the first 4 weeks (2 injections 4 days apart in the first week, and then one injection weekly). Blood samples from the jugular vein were collected in lithium heparin collection tubes (BD Vacutainer®, Becton Dickinson Co., Plymouth, UK) once per week to assess the plasma testosterone concentrations by radioimmunoassay (RIA); single assay; sensitivity 0.06 ng/ml; intra-essay CV 11.5%. Blood samples were centrifuged at 1900 g for 15 min and the plasma was immediately separated and stored at -20 °C until determination of the testosterone concentration by RIA.
Upon completion of the treatment, the animal was anesthetized once again, the above-mentioned examinations repeated, and a new attempt to collect the semen was made. The right testicle was found to have completed its descent into the scrotum and the urethral process had detached from the preputial mucous membrane (Figures 1B and 2B). The right testicle now had a normoechogenic echotexture, and had reached a diameter of 1.58 cm (the diameter of the left testicle was 1.97 cm) (Figure 3). The bulbourethral glands remained the same size, but the mean area of the seminal vesicles had increased to 0.26 cm2. The collected semen sample showed a very low sperm concentration, and most sperm were non-motile (oligoasthenospermia). Motility was assessed using a phase contrast microscope (Nikon Eclipse model 50i; negative contrast). Before treatment, plasma testosterone concentration was maintained on basal values, but reached a value of 8.0 ng/mL at 24 h after each hCG administration, falling again to basal values before the next measurement.
Figure 1: A) Before hCG Treatment the Urethral Process was Completely Adhered to the Preputial Mucous Membrane. B) After Treatment the Urethral Process had Detached from the Preputial Mucous Membrane.
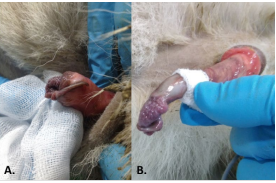
Figure 2: A) Cryptorchidism of the Right Testicle before Treatment with hCG. B) The Right Testicle Descended after Treatment with hCG.
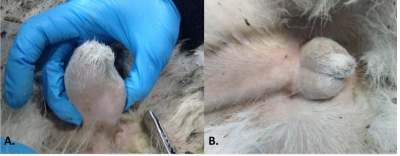
Figure 3: A) Left Testicle (LT) before Treatment with hCG. B) Left (LT) and Descended Right Testicle (RT) after Treatment with hCG.
This study was conducted according to procedures approved by the INIA Ethics Committee (reference number CEEA 2014/027), and appropriate guidelines were followed in accordance with the Spanish Policy for Animal Protection (RD53/2013), which conforms to European Union Directive 86/609 regarding the protection of animals used in scientific experiments. The INIA Ethics Committee approved the entire study.
DISCUSSION
Natural adhesions of the urethral process and glans penis to the prepuce are common in prepubertal lambs and goats.4 However, in a 2-year-old Iberian ibex male, it is a sign of sexual immaturity. In bovids, the urethral process becomes free under the influence of testosterone, and the penis separates from the preputial mucosa. This separation never occurs in early-castrated lambs4: the fusion between the urethral process and the preputial mucous membrane persists when lambs are castrated during the prepubertal period.5 This suggests that the rise in plasma testosterone in animals approaching puberty is involved in the aforementioned separation.
Plasma testosterone concentrations vary with season. In normal, mature ibexes, the testosterone concentration remains at baseline during winter and spring, but begins to rise in September, reaching a maximum (8 ng/mL) in October and November, before falling again to basal levels in January.3 Testosterone stimulates the growth of the vaginal process, the secretion of calcitonin gene-related peptide by the genitofemoral nerve (thus providing directional guidance to the gubernaculum), and then the regression of the gubernaculum, and the constriction of the inguinal canal.6 Males that produce insufficient testosterone suffer from reduced fertility, but in some mammalian species, this can be improved with hormone therapy.7,8 For instance, hCG can be used to increase follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels, which then stimulates the Leydig cells to secrete testosterone.8 After injecting hCG into bulls, plasma LH has been reported to increase after a few minutes, stimulating testosterone production.9 The present results suggests that in ibexes, the administration of hCG can induce a temporary increase in plasma testosterone, which can help the testes relocate to the scrotum.
Although, the post-treatment sperm concentration of the present ibex was low (oligospermia), and the sperm cells were mostly immotile, their simple appearance shows that hCG treatment stimulated spermatogenesis. The fact that treatment with hCG allowed the undescended testis to reach the scrotum, together with the increase in the size of both testes and the seminal vesicles, suggests that the sexual immaturity of this ibex was related to a gonadotrophin deficiency.
CONCLUSION
This is the first report of a sexual immaturity process in an ibex species with adhesions of the urethral process to the prepuce and cryptorchidism. hCG administration was effective to treat these alterations. More frequent treatment with hCG should be tested to determine whether full sperm functions can be recovered.
ACKNOWLEDGEMENTS
Grant MINECO AGL2014-52081-R.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.








