INTRODUCTION
Background and Rationale
In North America, approximately 80% of incident and 20-50% of prevalent hemodialysis patients receive hemodialysis via a central venous catheter (catheter).1,2 However, catheter use is associated with the highest morbidity and mortality of all vascular access types, primarily related to infection.3-11 Catheter related bacteremia is the most clinically important type of catheter related infection due to their common occurrence and potential to progress to sepsis and death.12 To prevent catheter related infection, patients should preserve the integrity and dryness of their catheter dressings.13-16 Wet dressings place patients at increased risk of infection, especially if their catheter exit site is not fully healed. Thus, it has been recommended to avoid showering, as it is difficult to attain full protective coverage of the exit site using dressings and barriers.
Despite this recommendation, patients inevitably continue to shower. In a survey of 274 catheter dependent hemodialysis patients, 64% indicated that the recommended prohibition to shower was moderately to extremely inconvenient and reduced their quality of life. Additionally 77% of patients admitted to showering at least once while they had a catheter.17 This is consistent with reports by hemodialysis nurses that patients often arrive to hemodialysis with wet and non-intact dressings due to showering.18 In the USA, there are limited catheter shower covers available for patient purchase and are cost prohibitive (e.g. $6-8.00 USD/shower use) to the majority of the hemodialysis population.2
To address the desire of patients to shower yet respect the associated infection risk, we developed a shower technique protocol aimed to minimize the risk of bacterial entry at the catheter exit site for patients with a fully endothelialized catheter tunnel and healed exit site. This shower technique protocol was designed to enable patients to shower and change their dressing on average, 3 times per week.
OBJECTIVES
Our long-term goal is to determine whether a formalized shower technique protocol could simultaneously allow showering to improve patient satisfaction with catheter care yet not increase their risk of catheter related infection. However, prior to designing and implementing the needed rigorous clinical trial to answer this question, we sought to determine whether such a study is feasible, with the following objectives:
Primary Objective: To determine if it is feasible to conduct a large multi-centre randomized control trial in satellite hemodialysis patients dialyzing via a catheter to test whether the catheter related bacteremia rate is non inferior in patients who use the shower technique protocol and standard care compared to standard catheter care alone over 6 months. Feasibility is defined by 5 outcomes, each with its own statistical test and measure of success; 4 of 5 outcomes must be achieved for the full study to be feasible (Table 1).
Table 1: Feasibility and Clinical Objectives for the HIPPO-SAT Pilot Study
|
Objective
|
Outcome Measure |
Criteria for Success (Feasibility) |
Method of Analysis
|
|
Primary Objective is the feasibility of the HIPPO-SAT study design defined by 5 outcomes below
|
|
1. To assess the accuracy of capturing the catheter related bacteremia rate in the study within the satellite hemodialysis setting
|
The level of agreement between the date the nurse contacts the study coordinator to inform them of a suspected infectiona and when the culture was sent to the lab |
Kappa level >0.80 |
Kappa statistic |
| 2. Determine the percentage of eligible hemodialysis patients who consent to participate |
The percentage of consented eligible patients in each satellite unit |
For each hemodialysis unit > 80% |
Percentages and confidence intervals
|
|
3. Determine the percentage of satellite hemodialysis patients with catheters who are screenedb
|
The percentage of satellite hemodialysis patients with catheters who are screened for eligibility |
For each hemodialysis unit > 95% |
Percentages and confidence intervals |
| 4. Measure the success of shower technique protocol teaching |
The percentage of patients in the intervention arm passing the Shower Technique Test at 3 and 6 months
|
>=80% of patients randomized to shower technique protocol |
Percentages and confidence intervals
|
| 5. Determine the percentage of participants in the control arm who are using aspects of the intervention |
The percentage of controls who are using aspects of the shower technique protocol which they were not using at baseline |
<5% participants in the control arm |
Percentages and confidence intervals
|
|
Secondary Objectives
|
Outcome Measure |
Criteria for Success OR Hypothesis |
Method of Analysis |
| Construct Validation of the Vascular Access Questionnaire (SF-VAQ)c |
The change in SF-VAQ score over time using the shower technique protocol compared to standard care. |
The shower technique protocol group will have a greater improvement in SFVAQ scores than the control group over 6 months |
Longitudinal regression model
|
|
Validation of the catheter exit site healing tests
|
The level agreement between the Deep Breath and Catheter Seal tests agree with the blinded photo test |
Kappa level >0.80 |
Kappa statistic
|
Secondary Objectives: To validate both the Short Form-Vascular Access Questionnaire (SF-VAQ) and the catheter exit site healing tests.
The results of the HIPPO-SAT pilot study will also allow for estimation of sample size requirements for the following clinical objectives of the potential future study:
Primary future study objective: To determine the catheter related bacteremia rates when using 1) the shower technique protocol and standard care, and 2) standard catheter care alone in satellite hemodialysis patients with healed catheter exit sites over 6 months.
Secondary future study objectives: 1) To compare the change in patient satisfaction with their vascular access over six months (measured by the SF-VAQ), using the shower technique protocol and standard care versus standard catheter care alone, and 2) To capture the costs associated with the shower technique protocol and standard care.
METHODS
Study Setting
This feasibility pilot study will take place in the satellite hemodialysis units affiliated with 2 academic centres, the University Health Network-Toronto General Hospital (Toronto, On.) and London Health Sciences (London, On.), and 3 community centres, the Scarborough General Hospital (Toronto, On.), Trillium Health Centre- The Credit Valley Hospital (Mississauga, On.), and Mackenzie Health Hospital (Toronto, On.). All 5 centres are located in South Central Ontario, Canada.
Trial Design
This study will be a multi-centre, single-blinded, pragmatic, randomized feasibility study for a larger clinical trial of the same design.
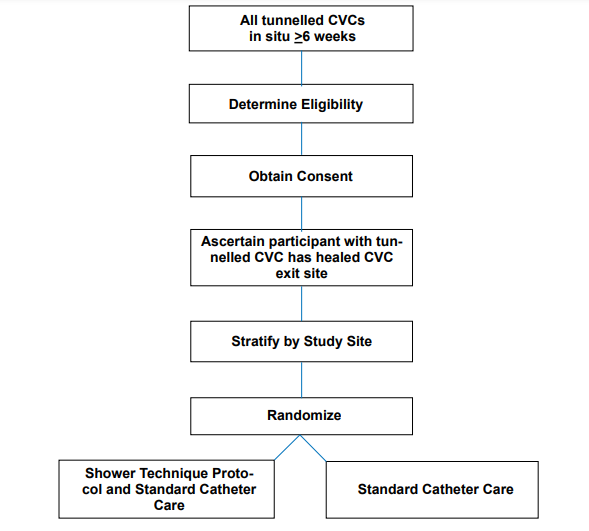
Population: The study population will consist of individuals requiring chronic hemodialysis who have a tunneled catheter in situ for longer than six weeks who meet the study inclusion criteria.
Inclusion Criteria: 1. Informed written consent obtained (English speaking); 2. Age >18 years old; 3. Requires a tunneled catheter as their vascular access: a) End stage kidney disease without a functioning surgically created vascular access; b) End stage kidney disease whose peritoneal dialysis problems require transfer to hemodialysis for an anticipated prolonged period; 4. Passes 2/3 tests of catheter exit site healing (see below); 5. Must be willing and able to take a shower as the standard form of body cleansing if randomized to receive the shower technique protocol; 6. Uses trisodium citrate (4%) as the standard catheter locking solution; 7. Their catheter has been in situ for > 6 weeks.
Exclusion criteria: 1. Acute kidney failure, likely to be reversible with recovery of kidney function; 2. Non-tunneled catheter; 3. Antibiotic use by any route in the week prior to enrolling in the study, including intranasal mupirocin; 4. On immunosuppressant therapy; 5. Use of the catheter for purposes other than access for hemodialysis; 6. Involvement in another interventional study related to their vascular access; 7. Patient life expectancy<6 months (e.g. active malignancy; serious comorbidity such as hepatic failure); 8. Routine use of intraluminal thrombolytic (e.g. recombinant tissue plasminogen activator) or antibiotic as a locking solution; 9. Catheter insertion in a location other than the neck/chest region.
Interventions
The planned trial intervention in participants with healed catheter exit sites will be either: i) training and use of the shower technique protocol when the participant wishes to shower plus standard catheter care or ii) standard catheter care provided by hemodialysis nurses at the satellite centre (Figure 1). The duration of the intervention will be 6 months from the time of randomization. If a participating site uses a prophylactic barrier at the catheter exit site as part of their catheter care protocol, they may only apply Polysporin Triple Ointment as a topical prophylactic agent, as per guideline recommendations.18 Patients will be taught to re-apply the Polysporin Triple Ointment according to standardized catheter care technique.
Figure 1: HIPPO-SAT Pilot Study Flow Diagram
Shower Technique Protocol Details of the shower technique protocol will be published with the results of the HIPPO-SAT pilot trial to prevent problems with participant ascertainment bias. Participants will be given a minimum 30 minute personalized educational session by the study coordinator. They will be taught safe and clean techniques for showering with their catheter. Video and other educational materials for the shower technique protocol will be used to assist in training participants randomized to this intervention. The participant must successfully demonstrate the shower technique protocol on a training mannequin (Shower Technique Test) and be deemed by the study coordinator as ready to independently and correctly perform the shower technique protocol before proceeding. If the participant passes the Shower Technique Test, they will be provided a pamphlet on the shower technique protocol, not to be shared with other participants, to be kept as a reference and placed in their bathroom/household. They will also be given the necessary supplies for the shower technique protocol, itemized in individual sequentially numbered kits, to take home. The study coordinator will track all these supplies for use in a separate cost analysis. Participants will be reassessed via the Shower Technique Test using the training mannequin 3 and 6 months following randomization.
Standard Catheter Care consists of cleansing with chlorhexidine 2% or povidone (if allergic to chlorhexidine) at the catheter exit site by trained hemodialysis nurses followed by placement of a dry gauze dressing by the hemodialysis nurse 1x/ week or when clinically indicated. Nuanced differences may be present at participating units; however, the key components of the intervention are 1) hemodialysis nurse delivery of catheter care 2) chlorhexidine or povidone cleansing 3) dry dressing 4) standardized frequency. Study coordinators will check dialysis run sheets monthly to confirm that nurse administered dressing changes are compliant with standard catheter care; non-compliance will be documented and reported to the Principal Investigator, vascular access coordinator and local nephrologist.
Outcome Measures
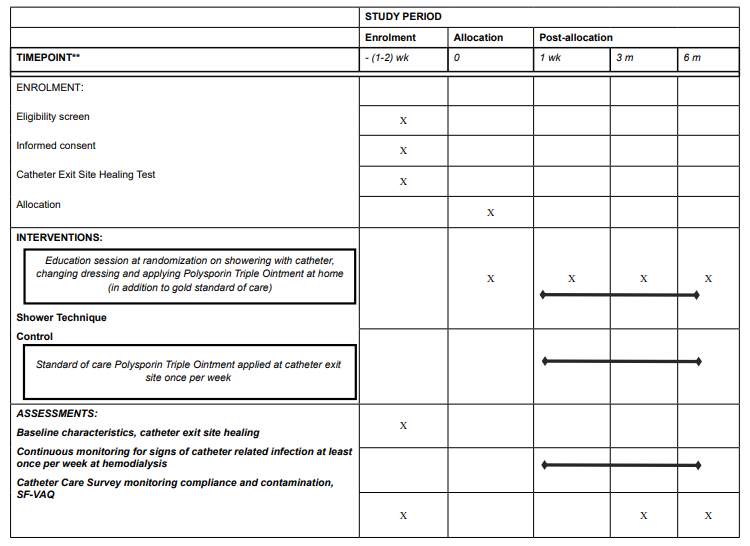
HIPPO-SAT pilot study objectives and their corresponding outcome measures are listed in Table 1. The primary outcomes for feasibility will be evaluated at the screening, recruitment, and implementation phases of the study. The catheter care survey, a measure of participant’s compliance and participant ascertainment bias with their catheter care protocol (also known as “study group treatment contamination”), and the SF-VAQ, a measure of vascular access specific satisfaction and quality of life, will be administered at baseline, 3 months, and 6 months post randomization.
The objective to assess the accuracy of the catheter related bacteremia rate capture in the satellite hemodialysis setting is critical for feasibility and will be described in detail. This will be measured by the level of agreement between the date the nurse contacts the coordinator to inform them of a suspected catheter related bacteremia and the date the culture was sent to the lab. The process for capturing the catheter related bacteremia rate is as follows: participants are clinically evaluated 3x/week on hemodialysis by their hemodialysis nurses, who are experienced at recognizing and managing patients with a suspected catheter related infection, including a catheter related bacteremia. The hemodialysis nurse will carefully inspect the participant’s catheter exit site and surrounding area at each catheter exit site dressing change. At the first sign of infection the nurse will notify a provider (e.g. physician) and the study coordinator immediately, take the appropriate swabs and blood cultures to obtain organism growth and sensitivities. The study coordinator will complete a data collection form (see Appendix 1) for each suspected infection which includes the date the nurse contacts the coordinator and the date the culture was sent to the lab.
Participant Timeline
The planned recruitment period is 12 months and the total duration of follow-up will be 6 months (Table 2). Study visits will take place at baseline, 3 months, and 6 months post randomization. Baseline clinical, demographic, and vascular access information will be obtained from the chart and/or a short interview with the participant. The catheter care survey and the SF-VAQ will be administered to all participants at each study visit. For those participants allocated to the shower technique they will receive their education session at baseline, and be administered the Shower Technique Test at each study visit.
Table 2: Schedule of enrolment, interventions, and assessments.
Supplies for the shower technique protocol will be distributed to patients once monthly.
Sample Size
This pilot study will help determine the sample size and analysis plan for the potential future larger study. The current sample size considers 50% eligibility, 30% refusal, 10% non-compliance and <1% loss to follow-up. The estimates are based on the following infection rates: the rate of catheter related bacteremia achieved with Polysporin Triple Ointment is 0.26-0- .63/1000 catheter days, and the catheter related bacteremia rate using shower technique protocol based on preliminary data is 0.39-0.46/1000 catheter days.18,19This preliminary data is derived from an informal shower technique protocol that was used in 49 select patients with no prior catheter related infection and who had used the same catheter for 6 months. Using the HIPPO-SAT’s broader inclusion criteria, the catheter related bacteremia rate may be higher. Data from each participating study site was also obtained to further support these estimates (data not shown). The planned recruitment target is 78 patients for the HIPPO-SAT pilot study.
Recruitment
The total study recruitment period will be 12 months from study initiation. Each study site will have 6 months to recruit participants; the study recruitment rate will be determined after the study recruitment period has ended. In order to protect against selection bias, all satellite hemodialysis patients with a catheter in situ for at least 6 weeks will be approached (see inclusion/exclusion criteria). A screening and recruitment log will be maintained and evaluated weekly during the recruitment period, to document reasons for exclusion and refusal.
METHODS: ASSIGNMENT OF INTERVENTIONS
Allocation
Once written consent is obtained, the participant will undergo formal testing for catheter exit site healing. If any of the tests are failed, standard catheter care will continue; the patient can be reassessed weekly until recruitment ends. Randomization will occur immediately after the eligible participant from whom consent has been obtained meets the entry criteria of “healed catheter exit site”.
Tests of Catheter Exit Site Healing
The tests of catheter exit site healing are as follows: 1) Deep Breath Stability Test measures the migration of the catheter as marked by a 2 cm indicator on the catheter from the skin at the catheter exit (test passed if<3 mm movement between complete exhalation and inhalation); 2) Catheter Seal Test is a visual inspection against an objective checklist to determine healing; and 3) Blinded Photo Test where two photos are taken of the catheter exit site to be evaluated for healing by independent blinded trained assessors (test passed if both assessors agree that the exit site is healed). These catheter exit site healing tests will be repeated at least weekly, or when the exit site is exposed, until 2/3 criteria are met or recruitment ends. These tests are only used on catheters that are at least 6 weeks old to ensure that all catheter exit sites are fully healed prior to patients being randomized to the study intervention arms.
Participant retention
In order to enhance patient retention and reduce loss to follow up, study coordinators are all trained in Good Clinical Practice guidelines i.e. to ensure proper patient selection and consent. Participants may rescind consent from the study at any time (e.g., if they find the shower technique protocol too challenging). Loss to follow up should be minimal as hemodialysis patients represent a “captive” study population due to their dialysis needs.
Allocation concealment mechanism
The participants’ necessary details will be provided to a 24-hour, telephone accessed independent central randomization desk (McMaster University) where each participant will be randomized and allocated to either the shower technique protocol and standard care or standard catheter care alone, and receive a corresponding unique study number. Allocation of patients to the intervention will be concealed to the randomization desk and will occur according to randomization sequence. The study coordinator will notify the participant of their allocation, and if randomized to the intervention arm, will immediately administer the shower technique protocol education session.
Sequence generation and implementation
Randomization will take place within strata formed by study site. The randomization schedule will be produced by a computer generated random number list, and will use a random permutated block design, with blocks sizes randomly selected. The central randomization facility (McMaster University) will know the randomization code. None of the study personnel or investigators will have direct access to the code.
Blinding
Patients and study coordinators cannot be blinded to allocation status due to the nature of the intervention; however, the outcome adjudication committee will be blinded to treatment allocation.
Statistical methods
The CONSORT criteria will be followed in the statistical analysis and reporting of this study. Study feasibility objectives, the corresponding measures of success and statistical tests are listed in Table 1. P-values < 0.05 will be considered statistically significant. All P-values will be two-sided and are unadjusted for multiple comparisons. All analyses will be based on an intention-to-treat approach. In sensitivity analyses, missing data will be imputed using multiple imputation methods. All analysis will use SAS v 9.4 and be carried out by a statistician blind to the intervention groups. There will be one analysis at the completion of this pilot study.
Monitoring of Trial Conduct and Procedures
To ensure conduct and reporting of the study that adheres to Good Clinical Practice, SPIRIT, and CONSORT guidelines, rigorous procedures have been put in place for the HIPPO-SAT pilot study. The procedures for data monitoring, obtaining and maintaining ethics board approval, protocol amendments are detailed below:
Data collection methods
All baseline and outcome data will be collected on paper data collection forms by the study coordinator, hemodialysis nurse, or Hemodialysis Infection Control Subcommittee member (Appendix 1 for example). They will then be entered into the computerized HIPPO-SAT pilot study database. There are no patient administered forms as the SF-VAQ and catheter care survey are both administered by the study coordinator to the patient.
Data management
All study data will be entered by the study coordinator into the HIPPO-SAT pilot study database. The HIPPO-SAT pilot study database is based on a Microsoft access platform and will be developed by a database developer and manager on the guidance of the study coordinator and the study statistician. It will be transferrable for analysis in the SAS (c) statistical program. A data dictionary will be created and maintained.
Data Safety Monitoring board and interim analysis
A Data Safety and Monitoring Board will not be required for this pilot study. However the study data will be continuously monitored for safety of the novel procedure. A Data Safety Monitoring Board will be assembled for the larger study, if this study is found to be feasible. There will be no interim analysis conducted in the pilot study.
Research ethics
Ethics board approval for this study has been obtained at the Scarborough Hospital, University Health Network – Toronto General Hospital, London Health Sciences Centre, Mackenzie Health Hospital, and at Trillium Health – The Credit Valley Hospital. In order to protect confidentiality before, during, and after the trial, personal information about potential and enrolled participants will be collected and maintained by the study coordinator at the study coordinating centre. The principal investigator, study coordinator, study statistician and monitors from the research ethics boards will be the only parties with access to the final dataset.
There are no relevant financial and other competing interests for principal investigators for the overall trial to disclose. The results of this study will disseminated at local, national, and international conferences and will be the final manuscript submitted to an indexed journal for publication. The local and principal investigators, as well as the trial steering committee will be included as authors in the final manuscript. No professional writers will be used to write the manuscript. (Appendix 2)
DISCUSSION
The inability to shower due to the potential increased risk for catheter related infection impacts on patients’ satisfaction with the presence and use of their hemodialysis catheter. While preliminary experience with a shower technique is encouraging18,20 it is critical to determine whether it is safe for use in patients with healed catheter exit sites (i.e. determine whether catheter related bacteremia rates using the shower technique protocol are not greater than with the gold standard of catheter care). It is also unknown whether using the shower technique protocol improves patient satisfaction with their catheter care. In non-randomized studies designed to answer these two important clinical questions there is a real potential for confounding as patients selected to use the shower technique protocol are likely to be those with minimal co morbidity, lowest infection risk, and highest level of compliance. Therefore it is critical that the new shower technique protocol be formally evaluated in a rigorously designed and implemented clinical trial prior to its widespread application.
However, prior to embarking on such a study, it is important to conduct a pilot study to address potential challenges associated with recruitment, consenting, catheter related bacteremia rate measurement, health care worker and patient compliance with the study interventions and protocol, and participant ascertainment bias in satellite centres. Each measure of feasibility in this pilot study tests whether these methodologic challenges are serious threats to the implementation of the potential future study. The most pressing concern that necessitates a pilot study are the issues of participant compliance and patient ascertainment bias (we refer to these as “study group treatment contamination”).
In the control arm of the HIPPO-SAT pilot study patients will be educated on the importance of not showering with their catheter in order to improve compliance. A recently conducted survey at the Toronto General Hospital and Scarborough General Hospital found that patients were 3.8 times more likely to comply with the recommendation not to shower if they remember being told not to do so by a healthcare professional.17 Patients randomized to the control arm will not be taught how to use the shower technique protocol, even if they admit to showering with their catheter. It is expected that a significant portion of patients in the control arm will shower against guideline recommendation, as per baseline; however, these patients are not considered crossovers as they are not using other aspects of the shower technique protocol, such as use of chlorhexidine swabs. As this is a pragmatic randomized trial design, both compliant and non compliant patients will be included in this study in order to reflect the clinical reality of hemodialysis patients in satellite units.
There is also potential for participant ascertainment bias in this study design where participants in the intervention arm share shower technique protocol techniques with the control arm participants. The control arm participants, however, will not have access to the training or necessary supplies required to properly perform the shower technique protocol -this will limit the impact of this bias. Where participant ascertainment bias exists, the extent of the problem will be measured in this pilot study. This is important for the external generalizability of the larger HIPPO-SAT study results because in non-study environments (i.e. clinical practice) there will be patients intermittently using the shower technique protocol within the same satellite unit. It is therefore necessary to understand the risks of participant ascertainment bias for those patients not using the shower technique protocol, which may be underestimated in the pilot study. Overall, the implication of this current pragmatic pilot design is to ensure future study feasibility and integrity while ensuring proper external validity and ultimately, greater practical generalizability to the real world setting.
CONCLUSION
The HIPPO-SAT Pilot study will determine whether a pragmatic randomized study design testing an educational intervention can be feasibly implemented in the satellite hemodialysis population. Statistical thresholds for capturing of the catheter related bacteremia rate, screening, recruitment, and shower technique education, as well as risk of participant ascertainment bias must be adequately achieved for the study to be deemed feasible. The secondary objectives of this pilot design is to validate both the catheter exit site healing tests and the SF-VAQ. The latter importantly addresses the need to balance patient preferences and satisfaction with their catheter care with health care provider’s concerns surrounding infection prophylaxis. With increasing patients with catheters in satellite hemodialysis, it is crucial that a pragmatic, yet effective, prophylactic catheter infection strategy be formally tested and established for this setting.
TRIAL SPONSOR: Kidney Foundation of Canada.
COORDINATING CENTRE: Toronto General Hospital is responsible for collection, management, analysis and interpretation of data.
TRIAL STEERING COMMITTEE: Lok C.E., Gafni A., Moist L., Thabane L.
ENDPOINT ADJUDICATION COMMITTEE: Batistella M., Bhola C
COMPETING INTERESTS
In the past five years have no author on this manuscript received reimbursements, fees, funding, or salary from an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future. None of the authors hold any stocks or shares in an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future. No author is declaring any patents relating to the content of the manuscript, or has received reimbursements, fees, funding, or salary from an organization that holds or has applied for patents relating to the content of the manuscript. No author has any other financial or non-financial competing interests competing interests to declare.
AUTHORS CONTRIBUTIONS
DK, CL, LM, and AG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. DK, CL, LT and AG participated in the design of the study and informed the statistical analysis approach. All authors read and approved the final manuscript.
AUTHORS INFORMATION
Dr. Charmaine Lok is director of the Renal Management Clinic and medical director of hemodialysis at the Toronto General Hospital. Dr. Lok is a Professor of Medicine at the University of Toronto and an Associate Professor in the Department of Clinical Epidemiology & Biostatistics at McMaster University.
ACKNOWLEDGEMENTS
This study is supported by the Kidney Foundation of Canada (KFOC). The KFOC had no role in the study design, collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.







