1. WHO. The World health report 2001 – Mental Health: New Understanding, New Hope.
Website: http://www.who.int/whr/2001/en/. 2001; Accessed 2015.
2. Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS et al. Grand challenges in global mental health. Nature. 2011; 475(7354): 27-30.
doi: 10.1038/475027a
3. Dixon J, Ferdinand M, D’Amico F, Knapp M. Exploring the cost-effectiveness of a one-off screen for dementia (for people aged 75 years in England and Wales). Int J Geriatr Psychiatry. 2015; 30(5): 446-452. doi: 10.1002/gps.4158
4. Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: A UK study. Aging Ment Health. 2015; 19(1): 63-71. doi: 10.1080/13607863.2014.915920
5. Russ TC, Gatz M, Pedersen NL, et al. Geographical variation in dementia: examining the role of environmental factors in Sweden and Scotland. Epidemiology. 2015; 26(2): 263-270. doi: 10.1097/EDE.0000000000000230
6. Murray CJL, Lopez AD. A Comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Global Burden of Disease and Injury Series. 1996, Harvard School of Public Health on behalf of the World Health Organization and the World Bank. 43.
7. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA, USA: American Psychiatric Association; 2013.
doi: 10.1176/appi.books.9780890425596
8. Pansari K, Gupta A, Thomas P. Alzheimer’s disease and vascular factors: Facts and theories. International Journal of Clinical Practice. 2002; 56(3): 197-203.
9. Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003; 22(1): 1-12. doi: 10.1159/000067110
10. Holstein J, Chatellier G, Piette F, Moulias R. Prevalence of associated diseases in different types of dementia among elderly institutionalized patients: Analysis of 3447 records. J Am Geriatr Soc. 1994; 43(4): 972-977. doi: 10.1111/j.1532-5415.1994.tb06590.x
11. Jackson GA, Dementia: Alzheimer’s disease. Nurs Times. 2002; 98(45): 32-34.
12. Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004; 140(7): 501-509.
doi: 10.7326/0003-4819-140-7-200404060-00008
13. Schwartz, S, Abnormal Psychology. Mauntain View. California, USA: Mayfield Publishing Company; 2000.
14. McKeel DW Jr, Price JL, Miller JP, et al. Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. J Neuropathol Exp Neurol. 2004; 63(10): 1028-1037. doi: 10.1093/jnen/63.10.1028
15. Zea-Sevilla MA, Fernández-Blázquez MA, Calero M, Bermejo- elasco P, Rábano A. Combined Alzheimer and cerebrovascular staging explains advanced dementia cognition. Alzheimers Dement. 2015; pii: S1552- 260(15)00059-X. doi: 10.1016/j.jalz.2015.01.004
16. Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015; 67(2): 195-203. doi: 10.1016/j.pharep.2014.09.004
17. Clark CM, Karlawish JH. Alzheimer disease: current concepts and emerging diagnostic and therapeutic strategies. Ann Intern Med. 2003; 138(5): 400-410. doi: 10.7326/0003-4819- 138-5-200303040-00010
18. Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ. Homocysteine, vitamin B-6, and vascular disease in AD patients. Neurology. 2002; 58(10): 1471-1475. doi: 10.1212/wnl.58.10.1471
19. Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004; 1000(1-2): 32-39. doi: 10.1016/j.brainres.2004.01.012
20. Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004; 3(3): 205-214.
doi: 10.1038/nrd1330
21. Mace S, Cousin E, Ricard S, et al. ABCA2 is a strong genetic risk factor for early-onset Alzheimer’s disease. Neurobiol Dis. 2005; 18(1): 119-125. doi: 10.1016/j.nbd.2004.09.011
22. Kan R, Wang B, Zhang C, et al. Genetic association of BACE1 gene polymorphism C786G with late-onset Alzheimer’s disease in chinese. J Mol Neurosci. 2005; 25(2): 127-132. doi: 10.1385/JMN:25:2:127
23. Zekanowski C, Styczyńska M, Pepłońska B, et al. Mutations in presenilin 1, presenilin 2 and amyloid precursor protein genes in patients with early-onset Alzheimer’s disease in Poland. Exp Neurol. 2003; 184(2): 991-996. doi: 10.1016/S0014-4886-(03)00384-4
24. Sobow T, Flirski M, Liberski PP. Amyloid-beta and tau proteins as biochemical markers of Alzheimer’s disease. Acta Neurobiol Exp (Wars). 2004; 64(1): 53-70.
25. Kowalska A. The beta-amyloid cascade hypothesis: a sequence of events leading to neurodegeneration in Alzheimer’s disease. Neurol Neurochir Pol. 2004; 38(5): 405-411.
26. Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008; 3(3-4): 115-126. doi: 10.1007/s12263-008-0091-4
27. Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev; 2012. 2012:914273. doi: 10.1155/2012/914273
28. Pavlica S, Gebhardt R. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic Res. 2005; 39(12): 1377-1390. doi: 10.1080/09670260500197660
29. Wang Q, Sun AY, Simonyi A, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005; 82(1):138-148. doi: 10.1002/jnr.20610
30. Borriello A, Cucciolla V, Della Ragione F, Galletti P. Dietary polyphenols: focus on resveratrol, a promising agent in the prevention of cardiovascular diseases and control of glucose homeostasis. Nutr Metab Cardiovasc Dis, 2010; 20(8): 618-625. doi: 10.1016/j.numecd.2010.07.004
31. Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010; 15(10): 7313-7352. doi: 10.3390/molecules15107313
32. Franz C, Chizzola R, Novak J, Sponza S. Botanical species being used for manufacturing plant food supplements (PFS) and related products in the EU member states and selected third countries. Food Funct. 2011; 2(12): 720-730. doi: 10.1039/c1fo10130g
33. Recio MC, AndujarI, Rios JL. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem. 2012; 19(14): 2088-2103. doi: 10.2174/092986712800229069
34. Schaffer S, Asseburg H, Kuntz S, Muller WE, Eckert GP. Effects of polyphenols on brain ageing and Alzheimer’s disease: focus on mitochondria. Mol Neurobiol. 2012; 46(1): 161-178. doi: 10.1007/s12035-012-8282-9
35. Lorrain, B, et al. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules. 2013; 18(1): 1076-1100. doi: 10.3390/molecules18011076
36. Naumovski N. Bioactive Composition of Plants and Plant Foods, in Plant Bioactive Compounds for Pancreatic Cancer Prevention and Treatment. In Scarlett CJ, Vuong QV. Nova Publishers: New York. 2015; 322.
37. Eschenauer G, Sweet BV. Pharmacology and therapeutic uses of theanine. Am J Health Syst Pharm. 2006. 63(1): 26, 28-30.
doi: 10.2146/ajhp050148
38. Vuong QV, Golding JB, Nguyen M, Roach PD. Extraction and isolation of catechins from tea. J Sep Sci. 2010; 33(21):3415-3428.
doi: 10.1002/jssc.201000438
39. Khan MA, Hussain A, Sundaram MK, et al. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol Rep. 2015; 33(4): 1976-1984.
doi: 10.3892/or.2015.3802
40. Shan HM, Shi Y, Quan J. Identification of green tea catechins as potent inhibitors of the polo-box domain of polo-like kinase 1. ChemMedChem. 2015; 10(1): 158-163. doi: 10.1002/cmdc.201402284
41. Nabavi SM, Daglia M, Moghaddam AH, Nabavi SF, Curti V. Tea consumption and risk of ischemic stroke: a brief review of the literature. Curr Pharm Biotechnol. 2014; 15(4): 298-303. doi: 10.2174/1389201015666140617100945
42. Li M, Hagerman AE. Role of the flavan-3-ol and galloyl moieties in the interaction of (-)-epigallocatechin gallate withserum albumin. J Agric Food Chem. 2014; 62(17): 3768-3775. doi: 10.1021/jf500246m
43. Koutelidakis AE, Rallidis L, Koniari K, et al. Effect of green tea on postprandial antioxidant capacity, serum lipids, C-reactive protein and glucose levels in patients with coronary artery disease. Eur J Nutr. 2014; 53(2): 479-486. doi: 10.1007/s00394-013-0548-0
44. Hagiwara M, Matsushita K. Epigallocatechin gallate suppresses LPS endocytosis and nitric oxide production by reducing Rab5-caveolin-1 interaction. Biomed Res. 2014; 35(2): 145-151. doi: 10.2220/biomedres.35.145
45. Cai J, Jing D, Shi M, et al. Epigallocatechin gallate (EGCG) attenuates infrasound-induced neuronal impairment by inhibiting microglia-mediated inflammation. J Nutr Biochem. 2014; 25(7): 716-725. doi: 10.1016/j.jnutbio.2014.02.012
46. Yang EJ, Lee J, Lee SY, et al. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1alpha with Th17/Treg control. PLoS One. 2014; 9(2): e86062. doi: 10.1371/journal. pone.0086062
47. Liu D, Zhang X, Jiang L, Guo Y, Zheng C. Epigallocatechin3-gallate (EGCG) attenuates concanavalin A-induced hepatic injury in mice. Acta Histochem. 2014; 116(4): 654-662. doi: 10.1016/j.acthis.2013.12.002
48. Apetz N, Munch G, Govindaraghavan S, Gyengesi E. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer’s disease–a translational perspective. CNS Neurol Disord Drug Targets. 2014; 13(7): 1175-1191.
doi: 10.2174/1871527313666140917110635
49. Nguyen P, Derreumaux P. Understanding amyloid fibril nucleation and abeta oligomer/drug interactions from computer simulations. Acc Chem Res. 2014; 47(2): 603-611. doi: 10.1021/ar4002075
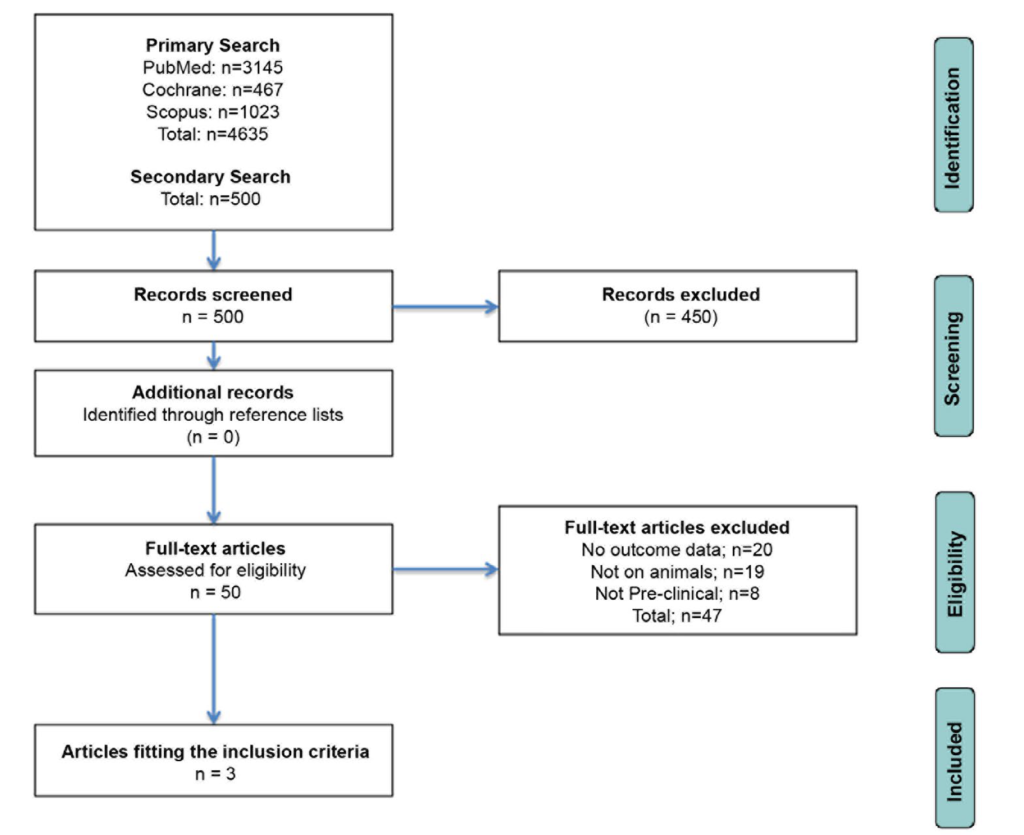
50. Lee HS, Jun JH, Jung EH, Koo BA, Kim YS. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules. 2014; 19(8): 12150-12172. doi: 10.3390/molecules190812150
51. Betts JW, Wareham DW. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014; 14: 172. doi: 10.1186/1471-2180-14-172
52. Zhao J, Suyama A,Tanaka M, Matsui T. Ferulic acid enhances the vasorelaxant effect of epigallocatechin gallate in tumor necrosis factor-alpha-induced inflammatory rat aorta. J Nutr Biochem. 2014; 25(7): 807-814. doi: 10.1016/j.jnutbio.2014.03.013
53. Reygaert WC. The antimicrobial possibilities of green tea. Front Microbiol. 2014; 5: 434. doi: 10.3389/fmicb.2014.00434
54. Gundimeda U, McNeill TH, Fan TK, et al. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: role of 67-kDa laminin receptor and hydrogen peroxide. Biochem Biophys Res Commun. 2014; 445(1): 218-224. doi: 10.1016/j.bbrc.2014.01.166
55. Saiko P, Steinmann MT, Schuster H, et al. Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dNTP pools and inhibit de novo DNA synthesis and proliferation of human HL-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine. Phytomedicine. 2015; 22(1): 213-222. doi: 10.1016/j.phymed.2014.11.017
56. Wei R, Zhu G, Jia N, Yang W. Epigallocatechin-3-gallate sensitizes human 786-O renal cell carcinoma cells to TRAILinduced apoptosis. Cell Biochem Biophys. 2014. doi: 10.1007/s12013-014-0428-0
57. Eom DW, Lee JH, Kim YJ, et al. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2014; pii: 2990. doi: 10.5483%2FBMBRep.2015.48.8.216
58. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010; 8(5): 336-341. doi: 10.7326/0003-4819-151-4-200908180-00135
59. Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J Nutr Biochem. 2008; 19(9): 619-626. doi: 10.1016/j.jnutbio.2007.08.008.
60. Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents Nmethyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001; 78(5): 1073-1082. doi: 10.1046/j.1471-4159.2001.00490.x
61. Rezai-Zadeh K, Arendash GW, Hou H, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008; 1214: 177-187. doi: 10.1016/j. brainres.2008.02.107
62. Saeed NM, El-Naga RN, El-Bakly WM, Abdel-Rahman HM, El-Demerdash E. Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: A mechanistic study. Biochem Pharmacol. 2015; pii: S0006-2952-(15)00110-0. doi: 10.1016/j.bcp.2015.02.006
63. Joshi R, Rana A, Gulati A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem. 2015; 176: 357-366. doi: 10.1016/j.foodchem.2014.12.067
64. Obregon DF, Rezai-Zadeh K, Bai Y, et al. ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallateinduced alpha-secretase cleavage of amyloid precursor protein.J Biol Chem. 2006; 281(24): 16419-16427. doi: 10.1074/jbc.M600617200
65. Lichtenthaler SF, Haass C. Amyloid at the cutting edge: Activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model. J Clin Invest. 2004; 113(10):1384-1387. doi: 10.1172/JCI21746
66. Dickey CA, Kamal A, Lundgren K, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007; 117(3):648-658. doi: 10.1172/JCI29715
67. Kosik KS, Shimura H. Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta. 2005; 1739(2-3):298-310.
doi: 10.1016/j.bbadis.2004.10.011
68. Khan SG, Katiyar SK, Agarwal R, Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992; 52(14):4050-4052.
69. McGahon BM, Martin DS, Horrobin DF, Lynch MA. Agerelated changes in LTP and antioxidant defenses are reversed by an alpha-lipoic acid-enriched diet. Neurobiol Aging. 1999; 20(6): 655-664. doi: 10.1016/S0197-4580(99)00050-0
70. Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem. 1998; 273(20): 12161-12168. doi: 10.1074/jbc.273.20.12161