INTRODUCTION
Gout results from the deposition of monosodium urate (MSU) crystals in joints and soft tissues. Gout was historically known as “disease of kings” or “rich men’s disease” and has been described since ancient Egyptians.1,2 It commonly occurs in the extremities, especially the first metatarsophalangeal joint. Systemic gout disease has been described in a limited number of cases.3,4 We present a case of a patient with systemic gout disease who did not receive any treatments for 10 years.
CASE REPORT
A seventy-one-year-old male came to our attention for physical weariness, chest discomfort, a mild fever for more than a month and right knee pain. Body temperature was 37.8 degrees and he had a premature heartbeat. He had no sore throat or nasal discharge suggestive of a cold. Past medical history revealed a 10-year right knee pain, but he had never sought medical attention and never got an accurate knee pain diagnosis. On physical examination, his right knee, right wrist and both feet were swollen. The skin overlying the right knee was red, suggestive of acute inflammatory change. He could move his right knee although he experienced knee pain. His blood test and laboratory data showed elevated WBC (9700/μL (normal 3500-9000/ μL)), UA (7.4 mg/dl (normal 3.0-7.0 mg/dl)) and CRP (1.34 mg/dl (normal <0.3 mg/dl)). Others findings were normal. We suspected polyarthritis, caused by gout from high serum UA. We performed 99mTc-MDP 740 MBq whole body bone scintigraphy to check where the polyarthritis was. The bone scintigraphy showed increased radioactive tracer suggestive of arthritis in the knees, feet and ankles, right wrist, sternoclavicular joints, and right ischium (Figure 1 arrows). In addition, questionable uptake of radioactive tracer was present in the sacroiliac joints, facet joints of L3 and L4 (Figure 1 arrowheads). Whole body CT scan showed multiple areas of bone erosion in sternoclavicular joints (Figure 2a), sternocostal joints (Figure 2a), right facet joint of L3 (Figure 2b), both sacroiliac joint (Figure 2c), right ischium (Figure 2d) and right knee joint (Figure 2e, 2f). Multiple soft tissue mass lesions suggestive of tophus were noticed in the facet joints of L3, the right ischial tuberosity and the medial condyle of the right femur and attachment of the right quadriceps femoris muscle tendon. Faint high density spotty lesions suggestive of deposition of MSU crystals were present in the sternoclavicular joints, sacroiliac joints and knee joint (Figures 2a, 2c, 2e and 2f). The CT scan revealed no malignant lesions in chest and abdomen. We performed a right knee arthrocentesis and found MSU crystals at polarized light microscope. The patient began to take a urate synthesis inhibitor (allopurinol) 200 mg for one day and his knee pain disappeared.
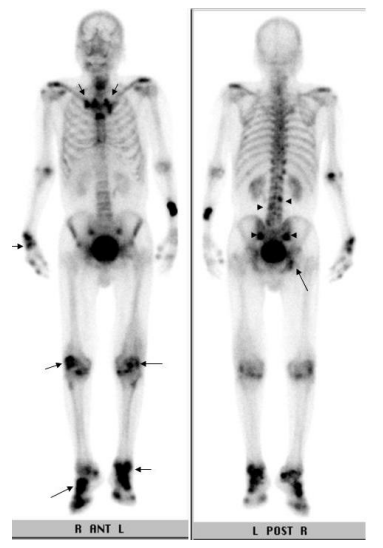
Figure 1: 99mTc-MDP 740 MBq whole body bone scintigraphy. Increased radioactive tracer is visualized in the sternoclavicular joints, sternocostal joints, knees, feet and ankles, right wrist, and right ischium (arrows). Spotty uptakes of the radioactive tracer are also demonstrated in the facet joints of L3 and L4 and sacroiliac joints.
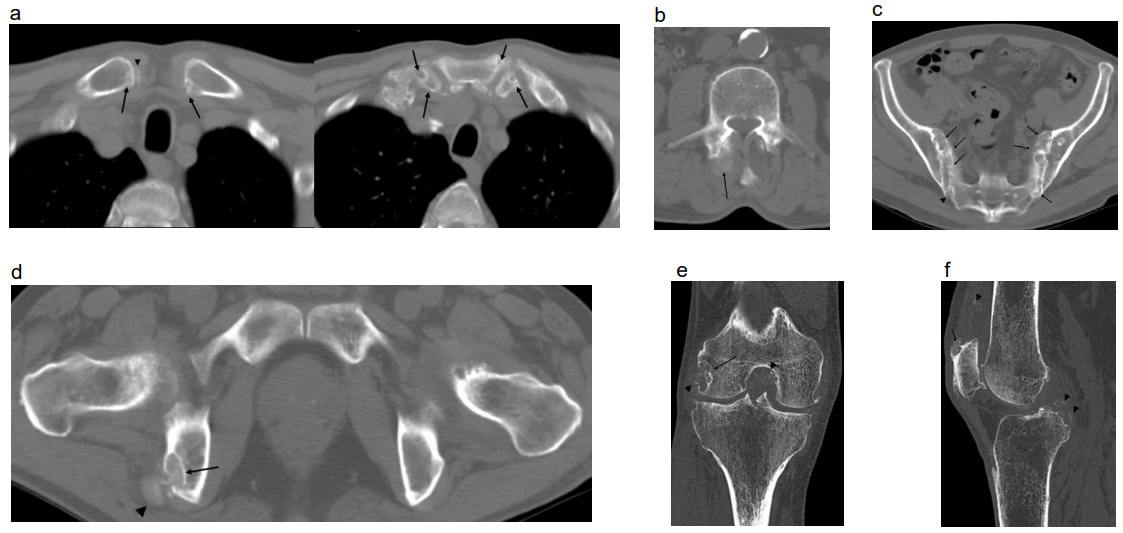
Figure 2a-f: Whole body CT.
a. Sternoclavicular and sternocostal joints: Multiple bone erosions with sclerotic rim are present in the both sternoclavicular and sternocostal joints (arrows). Calcification suggestive of the tophus is visualized within the right sternoclavicular joint (arrowhead).
b. Third lumber vertebral body: Faint calcification suggestive of the tophus is visualized in the right facet joint (arrow).
c. Sacroiliac joints: Diffuse bone erosion is visualized in sacroiliac joints (arrows). Slightly dense lesion suggestive of the tophus is present in the right sacroiliac joint (arrowhead).
d. Ischial tuberosity: Bone erosion (arrow) with the tophus (arrowhead) is visualized in the right ischial tuberosity. The left ischial tuberosity is normal.
e. Right knee joint (coronal image): Bone erosion called over hanging edge is present in the lateral condyle of the femur (arrow). Multiple calcifications suggestive of the tophi are visualized around the bone erosion and knee joint space (arrowheads).
f. Right knee joint (sagittal image): Oval shaped soft tissue lesion with spotty calcifications suggestive of the tophus is involved in the insertion of the quadriceps femoris muscle tendon (arrow). Similar calcifications are visualized both along the quadriceps femoris muscle tendon and the posterior cruciate ligament (arrowheads).
DISCUSSION
Gout is the most common form of inflammatory arthritis, with a prevalence of 3.9% in the USA (8.3 million individuals), 1.4- 2.5% (2.5 million individuals) in the UK and 1.4% (2.4 million individuals) in Germany.5 However, in Japan, the gout was a rare disease and it was first reported in 1898. In Japan, patients with gouty arthritis were about 80 in 1950’s, but they have been increasing since 1960.6,7 Today, approximately 0.8 million Japanese people have been receiving a treatment for gouty arthritis and/or hyperuricemia.3 Gouty arthritis typically affects the peripheral joints of the appendicular skeleton, especially feet and hands. On the other hand, gouty involvement of the axial skeleton is usually asymptomatic and typical radiological findings, such as erosive change and tophi, appear after several years.8 Bonaldi et al9 reported that 82% of the patients with spinal gout had chronic polyarticular gouty arthritis, tophi (mean duration, 14 years) and hyperuricemia. Our patient also had many polyarticular gouty arthritis and tophi including axial skeleton. All segments of the axial skeleton were involved by gout, but mostly the lumbar division. Alarcon-Segovia et al10 reported that sacroiliac joints were also involved. In our case, we confirmed gouty arthritis in the sacroiliac joints, sternoclavicular joints, sternocostal joints, and we detected tophi in the lumber spine and right ischial tuberosity on both bone scintigraphy and CT. This is a very interesting finding as there are no articles that mention ischial tuberosity tophi in the literature.
Regarding gouty arthritis in sternoclavicular joints, GR Sant et al11 in 1976 reported a case of an 18-year-old woman with throbbing pain, swelling and tenderness in the right sternoclavicular joint. They didn’t perform any arthrocentesis or biopsy for the detection of MSU crystals in the right sternoclavicular joint but they diagnosed gouty arthritis because of her hyperuricemia. We think that this was a misdiagnosis. Also Curry H,12 in his letter in response to their case report, stated that the level of uric acid in the blood cannot be reliably used to make a diagnosis of gout of the sternoclavicular joint, especially when the patient was ill and taking drugs. Authors in 1970’s could not perform CT for gout diagnosing, however, we were able to confirm at CT scan typical gout findings, like bone erosion and calcification nodules in sternoclavicular joints, even if we didn’t perform a biopsy.
Common causes of sternoclavicular joint abnormalities include osteoarthritis, infection, metastasis, primary bone/cartilage tumor, rheumatoid arthritis and radiation induced arthritis.13 Few authors reported sternoclavicular joint abnormality due to crystal deposition like MSU crystals.14 In patients with chronic gout, gouty arthritis in sternoclavicular joints needs to be considered as well.
CONCLUSION
Systemic gout disease affects not only the peripheral joints of the appendicular skeleton, but also the axial skeleton. Although joint aspiration is needed to detect MSU crystals, both scintigraphy and CT scan are powerful means to diagnose gouty arthritis at the same time. Gouty arthritis in sternoclavicular joints needs to be checked as well in patients with chronic gout.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
The patient has provided written permission for publication of the case details.







