CORE TIP
We report, fifteen years of experience in managing GISTs on a peripheral Portuguese Hospital. Surgery is the gold standard therapy. But in the adjuvant setting, recent studies have shown that imatinib, a tyrosine kinase, may change the natural history in high and very high risk tumors. On the other hand, recent molecular innovation has arisen concerning molecular characterization of these tumors, with direct consequences on the appropriate targeted treatment. Besides cost-effectiveness studies approved in treating specific groups of patients, this still continues as a heavy burden for the National Health Care system.
INTRODUCTION
Gastrointestinal Stromal Tumors (GISTs) are the most common mesenchymal (non-epithelial) tumors of the gastrointestinal tract. They probably originate from the interstitial cells of Cajal (ICC), which are located in the myenteric plexus of the gastrointestinal tract. ICC are the pacemaker cells responsible for gut peristaltic contractions.
They represent about 1-3% of all GI tumors, most of which 60% have gastric origin, and 30% comes from the small intestine. They are less common in the colon, rectum and esophagus (>1%). In other places within the abdominal cavity they can also be found, in less than 5%, in the omentum, mesentery or the retroperitoneum, and are known as extra-gastrointestinal stromal tumors (E-GISTs).
Historically, GIST’s were first described in the 1980’s, by Clark Mazur,1 when he introduced the first concept of GIST. The first mutation on the KIT oncogene was first documented in 1998, by Hirota.2 Until then, most GISTS’ were classified as leiomyomas, leiomyosarcomas or leiomyoblastomas.
Epidemiology
GISTs were in time considered an indistinct tumor, but are now considered a distinct neoplasm entity, with a particular histology, immunehistochemistry, molecular and oncogenic profile. Its worldwide incidence is estimated of around 1/1 00 000/year, and 10 cases per million in Europe.3 Prevalence rounds about 130 cases per million population.3 GISTs are more common in people older than 50 years old (>80%), rare under 20 years old (0-4%), and have similar frequencies in both sexes.
Clinical Presentation
Most GISTs are asymptomatic when small. Signs and symptoms are normally related to the location and size of the tumor. A mild gastrointestinal pain or discomfort can appear in 50-70% of the cases, GI hemorrhage in 50% cases, palpable tumor mass and also constitutional symptoms such as, anorexia, weight loss, fatigue, dyspepsia, dysphagia, nausea or vomiting, constipation or diarrhea and abdominal pain. Acute intraperitoneal bleeding leading to anemia or bowel perforation may also occur. At presentation, 15-50% of GISTS are metastatic. These are commonly found inside the abdominal cavity, namely in the liver, peritoneum and omentum and other sites.
Pathology
GISTs have three distinct histological patterns such as spindle cell, epithelioid and mixed. Being these patterns common with other tumors affecting also the gastrointestinal tract, immunohistochemistry markers are used to confirm the diagnosis. The most sensitive and specific markers for GISTS are CD117 (KIT) and DOG1 (ANO1-anoctamin 1) which are positive in more than 95% of GISTs. Only about 5% of GISTs are negative for KIT expression, but many of these are positive for DOG1. They also stain for CD34 in 70% of the cases, SMA (smooth muscle actin) in 15-60% and also stain for protein S100 around 10%. GISTs rarely express desmin.4
Risk Stratification of GIST
The risk stratification of GISTs is determined by analyzing three factors: tumor size, mitotic index and tumor location. This permits to characterize the risk of recurrence after surgery, and classify them in different groups: very low risk patients, low, medium and high risk patients, according to the model of “NIH” (National Institutes of Health).5 Patients with very low risk and low-risk tumors can perform only surgery; the intermediate risk and high risk may be indicated for adjuvant treatment. Emphasis on tumors where rupture of the tumor capsule occurs, always have indication for adjuvant treatment.
Oncogenic Pathway
In GIST, in 90% of cases occurring mutations in two oncogenes: the oncogene KIT (also known as CD117), where there are about 75-80% of the mutations, the most frequent of exon 11 and exon 9; PDGFRA oncogene (α receptor platelet-derived growth factor), where mutations occur in 10% of patients.
A sub-group of GIST’s, 10-15%, lack mutations in the oncogene KIT and oncogene PDGFRA. These are called wild-type GISTs. They englobe a heterogeneous group, which includes NF1 mutation, Carney-Stratakis syndrome, Carney’s v triad, BRAF mutations, succinate dehydrogenase subunit mutations (SDHA, SDHB, SDHC, SDHD) and RAS- family mutations too.
There may also be other changes, in particular in BRAF and NF1 (<2%) and SDH (succinate dehydrogenase) in approximately 10%. These subtypes of mutations have prognostic implications, including: point mutations in exon 11 of KIT oncogene (65%) which confers a favorable prognosis; mutations in exon 9 KIT oncogene (9%) are associated with poor prognosis; PDGFRA D842V mutations are associated with good prognosis in initial tumors, but the poor prognosis in metastatic GITS.
Treatment
According to an analysis of a pooled population-based cohorts, which included 2459 patients, estimated 5-year survival and 15 year recurrence-free survival rates for GIST treated with surgery alone was respectively 70.5% and 59.9% respectively.6 Since then, surgery has been considered the state of art for localized GISTs, as most patients with operable GISTs are probably cured. GISTs have been considered chemoresistance as has been demonstrated in several studies, were the response rate was less than 5% with a median survival for advanced disease was approximately 18 months.4 On the other hand, few data suggests that GISTs are sensitive to radiotherapy. It may have indication in a palliative situation, such as relief of symptoms, with a cumulative target dose of 30-50 Gy delivered in 2-3 Gy daily fractions.7 In the early 1990’s, Imatinib is a tyrosine inhibitor, was developed as a treatment for chronic myelogenous leukemia due to its capacity of inhibiting the fusion oncoprotein BCR-ABL. Due to structural similarities with KIT, several other experiments showed that imatinib can also inhibit the growth of cells that express mutant forms of KIT.8 Imatinib has been recommended for GIST tumors with KIT imatinib-sensitive mutations.
Early GIST
The drug of choice for treatment in the adjuvant setting has been Imatinib where positive results have been demonstrated in two randomized trials. An American trial, ACOSOG Z9001,9 713 patients were randomized into two arms (imatinib versus placebo); a statistically significant impact on recurrence-free survival in the imatinib group was demonstrated. In the European study AIO,10 400 patients with operable GIST were randomized with a high risk of recurrence in two groups: one received imatinib for 12 months and the other imatinib for 36 months. After five years, the results showed to be more favorable in the arm of patients treated for 36 months, concerning recurrence-free survival and overall survival.
Advanced GIST
In cases where a patient was treated with imatinib and developed metastases to the liver, one of the recommendations may be increasing the dose according to the patient’s tolerance and their comorbidities, where a good partial response or a stable disease may be accomplished.11
Sunitinib, is a second-line therapy, tyrosine kinase inhibitor, which is active in cells with mutations in exon 11 KIT and secondary exon 13 mutations (V654A) and exon 14 (T6701) of KIT, which confer resistance to imatinib, and, in fact, that demonstrated by a free time increase to progression (6.3 versus 1.5 months), a randomized trial of sunitinib versus placebo.12
Progression after imatinib and sunitinib can develop new mutations that confer resistance to these treatments. In this situation there are few alternatives. The use imatinib after a first approach with imatinib or sunitinib, in the study RIGHT (Rechallange of Imatinib in GIST Having the effective Treatment) did not lead to benefit in OS, but conducted to an increase in median PFS.13
For third-line treatments a new molecule appeared regorafenib, an oral, multikinase inhibitor which acts against KIT, PDGFR and VEGFR. It inhibits the tumor micro-environment (PDGFR, FGRF), proliferation of certain tumor cells (KIT, RET, RAF-1, BRAF, BRAF V600E) and also neo angiogenesis (VEGFR 1, 2, 3, TIE2). In the GRID study14 (Regorafenib in Progressive Disease phase III study design), 199 patients previously treated with imatinib and sunitinib, with metastatic unresectable GIST, were randomized (2:1) into two groups: one group of patients treated with regorafenib ID 160 mg every 21 days and best supportive care versus another group treated with placebo and best supportive care. There was a statistically significant increase in PFS (primary end point of the study), 4.8 months versus 0.9 months, with clear superiority of regorafenib arm, with a 73% reduction in the risk of progression or death. It was not demonstrated benefit of OS, but there was “crossedover” between the arms, the patient progressed on placebo were, many of them subsequently included in the regorafenib arm. The most common side effects were related regorafenib hand-foot syndrome, hypertension, and diarrhea. After this test, in August 2014, regorafenib is approved for use in metastatic GIST after failure of treatment with imatinib and sunitinib.
AIM
To evaluate fifteen years of experience in managing GISTs on a peripheral Portuguese Hospital. Define the behavior of GIST’s and the association between the histological, immunohistochemistry characteristics and disease progression. Question if the optimal treatment was delivered in GIST patients, based on medical evidence, where limitations on evaluating molecular signatures exists.
MATERIAL AND METHODS
Ethical Considerations
The study was reviewed and approved by the Oncology Depart ment of Centro Hospitalar entre Douro e Vouga, Institutional Review Board.
Data Analysis
A retrospective analysis of cases treated in Hospital of Entre Douro and Vouga from 1 January 1999 to 31 December 2014. Demographic characteristics were evaluated variables related to tumor and disease progression. All patients were evaluated in a multidisciplinary team. An expert treatment decision was made according to the National Institute of Health criteria of Gastrointestinal stromal tumor’s risk of recurrence after surgery.
Statistical Analysis
Statistical study was performed using SPSS version 21.
RESULTS
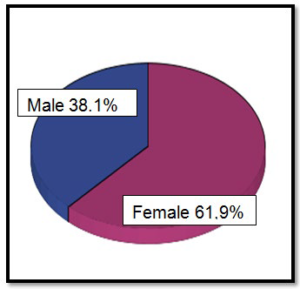
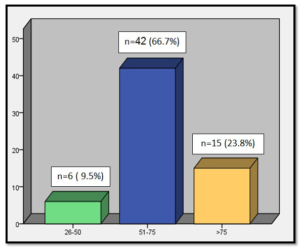
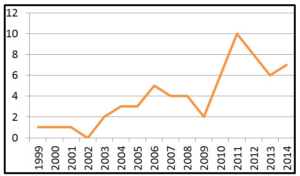
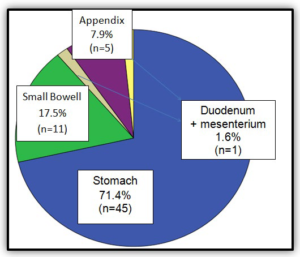
There have been 63 cases, 61.9% in female patients and 38.1% male (Graphic 1). The median age at diagnosis was 69 years (Graphic 2). As can be observed in (Graphic 3), a progressive increase in the incidence of GISTs was documented since 1999 to 2014. The most common source locations were: stomach (71.4%), small bowel (17.5%) and appendix (7.9%) (Graphic 4).
Graphic 1: Distribution by sex (n=63).
Graphic 2: Distribution by age.
Graphic 3: Incidence of GISTs during 15 years of experience.
Graphic 4: Tumor localization.
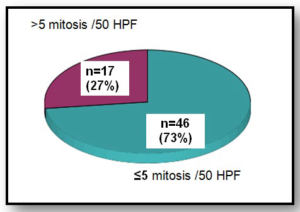
When assessing the Mitotic count (Graphic 5), 27% was superior to 5mitosis/50HPF and 73% was inferior to 5mitosis /50HPF. In total, 62 patients underwent surgery with R0 resection rate of 94%. No tumor rupture occurred either before or after surgery. R1 resection occurred in 4.8% (n=3) of the patients. One patient wasn’t submitted to surgery. Immunohistochemistry was performed in all patients, and sixty-one patients were positive for CD117. Only two patients were CD117 negative. No KIT gene mutation analysis was performed.
Graphic 5: Mitotic count.
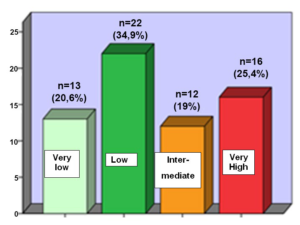
Regarding the biological risk of recurrence or metastasis, according to the National Institute of Health (NIH), 25,4 % of the patients had a very high risk, 19,0% had an intermediate risk, 34,9% had a low risk and 20,6 % had a very low risk (Graphic 6).
Graphic 6: Distribution of GISTs by the NIH classification.
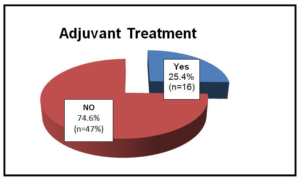
Of the 63 patients, 25.4% (n=16) were submitted to adjuvant treatment with imatinib (400 mg/daily) during 3 years (Graphic 7). Form this analysis 71.4% had tumors of very high risk, while only 12.2% had low risk.
Graphic 7: Patients under Adjuvant Treatment.
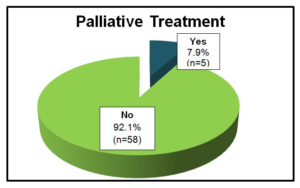
Only 7.9% (n=5) received palliative treatment with imatinib and sunitinib (Graphic 8). When analyzed, 3 patients were submitted to metastesectomy after progression, with an overall survival from a minimum of 4 to a maximum of 11 years until the last evaluation.
Graphic 8: Patients under Palliative Treatment.
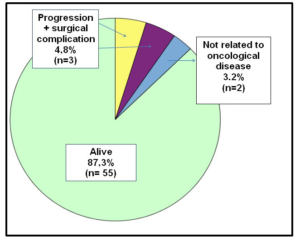
Two of the patients submitted to palliative treatment are alive, having survived from a minimum of four years to a maximum of 14 years. Three patients died after disease progression. In 11% of patients the disease progressed (median time to progression of 36 months). The mortality rate was 12.7% (n=8) and only three died of progressive disease. However, 55 patients were alive (87.3%) at the end of this retrospective study (Graphic 9).
Graphic 9: Results.
DISCUSSION
Strengths and Weaknesses of the Study
The mutation analysis is critical in the clinical decision on adjunctive therapy, as with the cases of the KIT exon 9 mutation may respond favorably to the increase in dose of imatinib, while the genotypes PDGFRA D842V mutations are less sensitive or resistant to imatinib.
In our Hospital the above-mentioned mutations were not studied, which might have influenced the treatment and prognosis of our patients. Nevertheless, an increase of diagnosis of GIST was observed since 1999, as physicians became more aware of this new entity. The survival rate of patients studied was high (87%). This may be justified by the fact that most of the patients were very low or low risk (55.5%) and also to the fact that in 94% of the cases, R0 resection was accomplished. It was found that only patients who had high biological disease risk, showed progression and mortality associated with cancer disease.
FUTURE SRATAGIES
Based on pharmaco-economic studies recently published in Oncologist15,16 it has been emphasized that adjuvant treatment with imatinib must not be neglected, based on mutational analysis and dose administration. This is justified by a significant economic impact on the national health system, and on the other hand, its adequate use concerning dosage and mutational status, allows a better approach to cost-benefit level for each patient.
On a palliative point of view, GISTs’ recurrence is also associated with an economic and social cost that must be brought up.17 The optimization of the therapeutic target in the treatment of GISTs will provide an overall benefit concerning the patient and its physician, with a tailored molecular therapy and life-saving approach. More pharmoco-economic studies focusing the importance of further molecular characterization of this disease must be supported and carried out, not only for caresaving but also for health-saving of the national health system.
ACKNOWLEDGEMENTS
The authors thank to the participating team involved in elaborating this study directly and indirectly.
AUTHORS CONTRIBUTION
Joana Espiga de Macedo and Pedro Santos contributed to study conception, design and writing article; editing and reviewing; Sílvia Lopes and Mónica Pinho contributed to date acquisition, data analysis and interpretation; Joana Espiga de Macedo contributed for the final approval of the article.
SUPPORTED FOUNDATIONS
Joana Espiga de Macedo and the Department of Medical Oncology, Centro Hospitalar de Entre o Douro e Vouga, Portugal.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was reviewed and approved by the Department of Medical Oncology, Centro Hospitalar de Entre o Douro e Vouga, Institutional Review Board.
INFORMED CONSENT
All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
CONFLICTS OF INTEREST
Joana Espiga de Macedo has received fees for serving as a speaker, such as consultant and/or an advisory board member for Celgene, Merck and Roche. Silvia Lopes, Mónica Pinho and Pedro Santos have no conflict-of-interest.














