CASE REPORT
A 30-year-old female with a 3-year history of Crohn’s Disease (CD) of the terminal ileum and complaining recently of a 3-week progressive abdominal pain, nausea and diarrhea (5-6 bowel movements/day) was admitted to the hospital. The patient was febrile (37.5 °C) and presented right lumbar pain radiating to the gluteus region. The patient had been treated with methotrexate, mesalazine and metronidazole over the previous 6 months.
Upon presentation, the abdominal examination revealed normal bowel sounds and abdominal tenderness without rebound. A mass, 7 cm by 3 cm, was palpated in the lower right quadrant, which was tender to palpation, without guarding, rebound or tap tenderness. The remainder of the examination was normal. Blood tests showed moderate leucocytosis and an increase both in Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP) levels were observed. Results are shown in Table 1.
| Table 1: Laboratory data |
| Erythrocyte count (x106mm3)
(range 3,800-4,800) |
3,750 |
| Hematocrit (%)
(range 34-42) |
38 |
| Hemoglobin (g/dl)
(range 11.5-13.5) |
11 |
| White-cell count (mm3)
(range 5,000-9,000) |
10,500 |
| Neutrophils (%)
(range 30-65) |
87 |
| Lymphocytes (%)
(range 30-48) |
22 |
| Platelet count (mm3)
(range 150,000-450,000) |
345,000 |
| Mean corpuscular volume (µm3)
(range 77-95) |
87 |
| Erythrocyte sedimentation rate (mm/hr)
(range 0-25) |
65 |
| C-reactive protein (mg/dl)
(range 0.01-0.05) |
18 |
Magnetic Resonance (MR) enterography was performed which revealed thickness of the terminal ileum wall, with associated fistulae from the ileocecal valve to the retroperitoneal and retrofascial space (Figure 1-3); The presence of a large fluid collection was detected in this area. Computed Tomography (CT) was performed to further investigate these findings which revealed, in the retrofascial space, an abscess measuring 6 cm by 5 cm filled with air and fluid (Figure 4).
Figure 1: A 30-year-old female with a 3-year history of Crohn’s disease of the terminal ileum. Coronal MRI (GE Echospeed 1.5 T HD), T1 post-gadolinium (Multi- Hance) demonstrates thickening of the ileum wall and perivisceral fistulae.
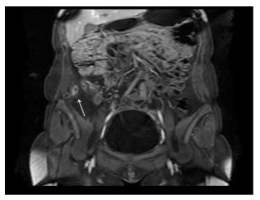
Figure 2: A 30-year-old female with a 3-year history of Crohn’s disease of the terminal ileum. Coronal plane MRI (GE Echospeed 1.5 T HD), T1 post-gadolinium (MultiHance) demonstrates an abscess in the right retroperitoneal space, below the right kidney.
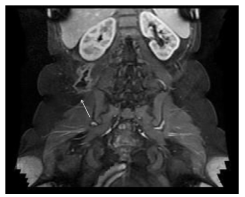
Figure 3: A 30-year-old female with a 3-year history of Crohn’s disease of the terminal ileum. Axial MRI (GE Echospeed 1.5 T HD), T1 post-gadolinium (MultiHance) demonstrates an abscess in the right retroperitoneal and para-vertebral space with enhancement of the walls.
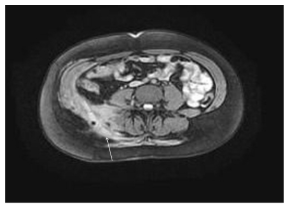
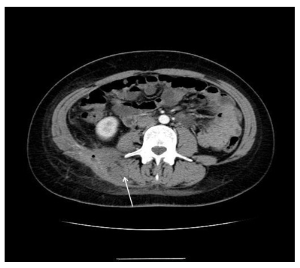
Figure 4: A 30-year-old female with a 3-year history of Crohn’s disease of the terminal ileum. Axial CT with contrast (Ultravist 370) in the portal venous phase (GE Optima 660, 1.5 mm slice thickness, 120 mVp, 200 mAs) demonstrates thickening of the right para-vertebral muscles containing a gas bubble.
Immunomodulators were immediately withdrawn and antibiotic therapy was started administering intravenous (iv) metronidazole and ciprofloxacin. The patient underwent abdominal surgery. An ileocolectomy was performed with a latero-lateral ileocolonic anastomosis. Unfortunately, when the surgeons performed the laparotomy they weren’t able to localize the collection which was deeply located in the retroperitoneal space, close to the spinal column. So, after the surgery, the patient underwent an ultrasound study which revealed thickening of the right para-vertebral muscles and a hypoechoic abscess in the posterior soft tissue. Thereafter, a CT-guided percutaneous drainage of the retroperitoneal abscess was performed and approximately 300 ml of purulent fluid were aspirated and a drain was placed therein. Ampicillin, gentamicin and metronidazole were administered iv. Gram’s staining of the aspirate revealed abundant polymorphonuclear leukocytes, gram-negative and gram-positive rods and moderate gram-positive cocci in pairs.
Following surgery and percutaneous drainage, body temperature returned to normal within 3 days. Six weeks after discharge, immunosuppressive therapy was reintroduced with good clinical results.
DISCUSSION
CD is a chronic inflammatory disorder which may involve any segment of the Gastrointestinal (GI) tract and which is characterized by trans-mural damage of the bowel wall.1 The incidence of CD is approximately 5-10 new cases per 100,000 individuals/year and is known to follow an unpredictable course with periodic exacerbations.2 Although the terminal ileum is the most common site of CD and upper GI involvement is rare, multiple non-contiguous segments of the small bowel may be involved.3 Since CD is characterized by a trans-mural inflammatory process, leading to symptomatic strictures, the differentiation between a prevalent inflammatory or fibromatous process is often difficult but is essential for appropriate clinical management (i.e., medical vs. surgical treatment).4
MRI provides a definite soft tissue contrast resolution enabling a good visualization of the inflammatory and fibrotic characteristics of the bowel wall. Normal bowel thickness is considered to be ≤3 mm.5 With time, common complications of small-bowel CD may include bowel obstructions, fistulae and abscesses.
Fistulae may act as a bridge to adjacent loops of the small bowel or from the small bowel to the colon, stomach, bladder and skin. In population based studies, fistula formation has been reported in 17-50% of patients with CD.1,2 According to an epidemiologic study, 35% of the CD patients develop at least one fistula episode during the course of the disease. Of these fistulae, approximately two thirds are external (perianal 55%, enterocutaneous 6%) and one third are internal.2 Penetrating disease may cause the formation of abscesses which can often be treated by a percutaneous approach. The presence of penetrating disease, in the absence of a collection, often influences the medical approach; clinicians, usually avoid the use of steroids in such cases and may, instead, consider antibiotic or biologic therapy.
Fistulae occur as the result of deep transmural ulcers or fissures that eventually penetrate the bowel layers and cause inflammation in the adjacent mesenteric tissue leading to formation of small abscesses and blind-ending sinus tracts. Upon CT and MRI, fistulae appear as extra-enteric enhancing tracts which may or may not contain fluid.
Recent advances in CT and MR imaging (MRI) offer excellent visualization of the small bowel wall and lumen when the organ is appropriately distended using an enteric contrast. In particular, these techniques can reveal small bowel inflammation and complications (e.g., strictures, fistulae, abscesses).6 Furthermore, MRI is becoming more and more important as a diagnostic tool in the work-up of CD, given the lack of ionizing radiations, considering that these examinations are performed frequently during the follow-up of these patients. Several studies have also shown that the MRI technique defines not only the site but also the degree of CD activity, by evaluating the increased enhancement of the involved bowel wall by means of contrast T1-weighted sequences.6,7,8,9,10,11,12 Horsthuis et al. performed a meta-analysis and reported that there was no statistically significant difference between MRI and CT in the detection of inflammatory bowel disease, including extra-enteric complications.13
The retroperitoneal abscess is a relatively rare condition and is often misdiagnosed or even recognized late in the clinical course. It is a severe infection which is associated with significant mortality but early diagnosis and treatment considerably reduce the morbidity and mortality rates. Recognition of the condition and the likely etiological factors would increase awareness and, therefore, early surgical drainage would significantly improve clinical outcome.
Herewith, we presented an illustrative case of fistulizing CD complicated by a retro-peritoneal abscess. The retroperitoneum is a space between the peritoneum and transversalis fascia which lines the posterior abdominal cavity. It extends laterally to the edges of the quadratus lumbar muscles, the diaphragm above and the pelvic brim below. The retroperitoneum is divided into an anterior space containing the colon, duodenum, and pancreas and a posterior space in which kidneys, aorta, and inferior vena cava are found. The area is closed above but is open below thus allowing retro-peritoneal infections to be bilateral and extend to the pelvis and thighs. The retrofascial space which is located between the transversalis fascia and the posterior parietal wall contains the spinal and para-vertebral musculature. This area extends above into the mediastinum and below into the pelvis and thighs. Infections of the retrofascial area that are not strictly in the retroperitoneal space are included in the differential diagnosis of the retro-peritoneal abscess. Thus, infections and inflammatory processes of any of these anatomical structures may result in a retro-peritoneal abscess. Abscesses resulting from deep fissuring of the bowel wall or suppuration within lymph nodes and local fistulation, develop in approximately 20% of CD patients.1,2 Several cases of vertebral and para-vertebral infection have been described in association with enteric fistulae.14 Isolated discitis, however, has rarely been reported.
In conclusion, we describe the unusual case of a hidden retroperitoneal abscess with para-vertebral collection as a consequence of a penetrating CD of the terminal ileum, emphasizing the importance of complete radiological work-up in these patients and the need for a careful clinical and surgical evaluation in order to define the most appropriate medical treatment in such cases.
AUTHORS’ CONTRIBUTIONS
Renato Caviglia and Basilio Lippi contributed equally to this article. Angelo Di Castro and Claudio Cannaviello collected the medical data and read and approved the manuscript.
ACKNOWLEDGEMENTS
The Authors are grateful to Mrs. Marian Shields for helping with the preparation of the manuscript as well as the language.
DISCLOSURES
The authors have no conflicts of interest to declare.
CONSENT
Did the authors obtain written informed consent from the patient for submission of this manuscript for publication? Yes









