INTRODUCTION
Crustaceans are found in aquatic, terrestrial, and aerial forms; they have jointed appendages or legs; they are triploblastic and bilaterally symmetrical; and the body is divided into the head, thorax, and abdomen.1 There are three major kinds of parasitic crustaceans affecting commercially important aquaculture species, the majority of which are external parasites: copepods (Ergasilidea and Lernaeidae), Branchiura (Argulidae), and isopods of Branchiura.2,3 Parasitic crustaceans are found globally in fresh, brackish, and salt waters. Many parasitic species have free-living larval stages.4 Copepods have been parasitic on fish since the lower Cretaceous period, perhaps 110 to 120 million years ago. Almost 30 copepod groups have parasites that use fish as hosts, with the majority found exclusively on fish.5
Most freshwater parasite crustaceans can be seen with the naked eye as they attach to the host’s gills, body, and fins.6 Small crustaceans or copepods are common parasites on the gills of aquarium and pond fish, causing gill obstruction and fish death from oxygen deprivation.7 Copepods have two primary life cycles: Nauplius and Copepodid.8 The Branchiura consists of approximately 175 species, divided into four genera and put into a single family, the Argulidae. Argulus (fish lice) have five limb-bearing segments, a trunk separated into a thoracic region containing four pairs of powerful swimming legs, and a short abdomen with a single bi-lobed unit caudal fin.9 Argulus, sometimes known as fish lice, is a common Branchiura parasite found in fish that has also been found in frog tadpoles. It is a big ectoparasite that may migrate across the body surface of the fish. All life stages of both sexes are parasitic.10 Isopods are widely seen on teleosts in tropical and subtropical waters, clinging to the body surface, in the mouth, or on the gills. The body is divided into three sections: the head, which is unsegmented and carries two pairs of antennae; a pair of variable- sized eyes; and a mouth. Most of them are ectoparasites.11 One of the key characteristics of parasitic isopods is that they are often maritime and prefer warmer oceans.12 They inflict harm similar to other copepods, but the most significant effect of isopod infection is the loss of host tissue caused by the parasite’s body pressure.13 The cymothoidae life cycle involves only one host (the Holoxenic cycle), and they connect to fish early in life and go through a male stage (cymothoids are protandrous hermaphrodites).12
When working with fish, disease prevention is usually more satisfying than treatment. As a result, avoid acquiring or introducing fish to a freshly constructed tank that demonstrates aberrant swimming behavior. Signs of fin and body injuries.14 A complete fish health management program should, in most situations, be founded on the concepts of water quality, nutrition, sanitation, and quarantine Khan et al.15 There are many reviews published on parasites in livestock, but there are few reviews published on fish parasites. Therefore, the main objective of this review is to provide an overview of the ectoparasites of fish.
CRUSTACEAN PARASITES
There are three major Ergasilidea kinds of parasitic crustaceans affecting commercially important aquaculture species, the majority of which are external parasites: copepods (Ergasilidea and Lernaeidae), Branchiura (Argulidae), and isopods (Branchiura).15 These parasites are numerous and found in fresh, brackish, and salt-water aquaculture systems all over the world. Branchiura (Argulidae) and isopods with parasitic sexes are larger than copepods, which are normally tiny to microscopic.16
Crustacean parasites have three bodily parts: the head, the thorax, and the abdomen. These zones are difficult to differentiate due to the merging of diverse sections. Branchiura (Argulidae) and isopods with parasitic sexes are larger than copepods, which are normally tiny to microscopic.6,17
When present in modest quantities, they usually inflict very minor damage to their hosts. However, in the case of severe infections, serious damage to skin, muscles, and gill tissue, as well as secondary infections, can develop.2 Many parasitic crustaceans have free-living larvae.4 Most freshwater parasite crustaceans are visible to the naked eye as they adhere to the host’s gills, body, and fins.6
Branchiura species show wide-ranging morphophysiological differences, allowing them to exist in a variety of habitats and lifestyles, ranging from planktonic species to parasites.18-21 However, parasitic species require a host at least once during their life cycle. In contrast, ergasilids vary in their amount of host specificity; some are only exclusive to their host genus (for example, ergasilids infecting Cichlidae). Others are more opportunistic in their host selection.2
Copepods are the most common crustacean fish parasites, and male parasitic copepods die after copulation in the preadult stages; thus, those seen attached to fish are usually mature females with conspicuous paired egg sacs at the posterior end.3,16
COPEPODS
Copepods have been parasitic on fish since the lower Cretaceous period, perhaps 110 to 120 million years ago. Almost 30 copepod groups have parasites that use fish as hosts, with the majority only found on fish.5 The Lernaeidae family includes freshwater parasites that are highly adapted to a parasitic lifestyle, cephalothoraxes that account for half or more of the body length, the parasite body part is segmented with the thorax (except the first segment, which is fused with the head), and the abdomen is distinct.16,22 Given the worldwide distribution of anchor worm-infected fish,23,24 L. cyprinacea infection can have a deleterious impact on host dynamics at the individual, population, and community levels.25 Only sub-adult and adult females exist in fish gills, and a few genus paraergasilus are attached in places other than gills.26
Characteristics of the Copepod
The connection of copepods with biotic aspects of the aquatic environment is traced through topical, trophic, behavioral, and parasite-host relationships with other species. Copepods serve a significant ecological function in the transmission of parasites found in vertebrates as ultimate hosts. Copepods also have an important ecological role by acting as bastions (habitat) for various suctorians, chonotrich and peritrich ciliates, fungi, and bacteria. Seasonally, the prevalence of copepod species was highest before heavy rain and lowest during the monsoon. However, P. varunae is more common before heavy rain. During the post-heavy rain season, all copepod species had a high prevalence.27 Copepods’ potential effects on their fish hosts are difficult to quantify, especially in wild fish like.28 Despite this, the prevalence of many parasites changes greatly from season to season. Investigations into the seasonality of parasite incidence were still limited.29
The Life Cycle of a Copepod
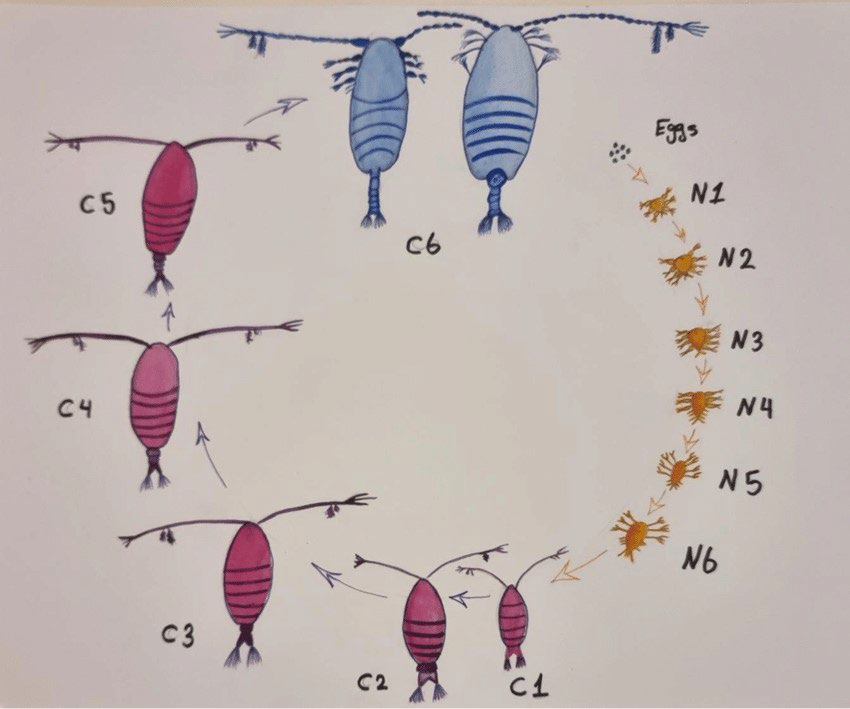
Copepods have two primary life cycles: Nauplius and Copepod. The egg usually hatches into a Nauplius larva, which has a tiny, unsegmented body and only three pairs of functional appendages: antennules, antennae, and mandibles. There are a maximum of six Nauplius stages (designed NI-NVI: Nauplius stage one up to stage six), and all six are retained in most free-living copepods and some parasitic copepods. Nauplius can be planktotrophic, feeding on other planktonic species, or lecithotrophic, feeding on yolk reserves. Lecithotrophic Nauplius is distinguished by the reduced station on the three limb pairs and the absence of the so-called Nauplius feeding process on the antenna’s coxa. The last nauplius stage (NVI) also experiences a metamorphic molt to the first copepod, which includes a segmented body, a full adult set of cephalic appendages, and the first and second swimming legs.8
There is a maximum of five copepod phases (named CoI-CoV: Copepod stages one up to Copepod five) in free-living copepods, and one body somite is added at each molt through this phase. The fifth copepod stage molts into an adult in both sexes.30
This is the last or definitive molt, and the female becomes sexually receptive to molting. Mating occurs shortly after the female becomes sexually receptive, and adult males may participate in precopulatory mate guarding, holding pre-adult females until the final molt.
Only the female Ergasilidae is parasitic and lives on the gills of fish. Males are free-living, and larval development includes three to six stages of nauplii and four to six stages of copepodites (lasting from 10-days to over a month). Free-living stages feed on nanoplankton.2 The quantity of eggs (20-100) varies by species as well as age and metabolic health, which are controlled by the attachment position on the gills. Temperature-dependent hatching time is 3 to 6-days at an ideal ambient temperature. Elevated salinity has been shown to slow larval growth.22
Females linked to the gills lay eggs in two sacs attached to the vaginal region. Females undergo metamorphosis, insert their anterior body into the host tissue, and then generate eggs when males mate on the fish host and die.30,31.32
Lernaeidae (anchor worms) have a complicated life cycle with multiple larval stages, each of which is a molt.16 There are three nauplii, five copepod phases, a pre-adult stage, and an adult stage. Copepods and adult females were discovered on the same host, implying that at least some can complete their life cycle on a single host. In lower temperatures, just one generation may occur every year, whereas in warmer areas, the life cycle might be completed in 12 to 14-days (Figure 1).4
Figure 1. The life cycle of Copepod, Source: The eggs hatch and develop through 6 naupliar stages (N1-N6) and then 5 copepodite stages (C1-C5) before becoming adults (C6) Abdulhussain A.33
Pathogenesis Copepods
Small crustaceans, or copepods, are common parasites on the gills of aquarium and pond fish, causing gill obstruction and fish death from anoxia.7 Copepods were grazing on gill tissue, and their feeding activity was linked to gill damage such as epithelial hyperplasia, telangiectasis, and hemorrhage. Copepodites can damage and necrotize the epithelium of fish gills or the buccal cavity. Parasitic copepods cause direct harm to their hosts through attachment mechanisms and feeding activities. Ectoparasites attach via clawed limbs, and penetration of the skin by the claws generates local lesions, the pathophysiology of which varies depending on size and other parameters.14
The severity of these diseases is determined by the following factors: the life stage, species, and number of crustaceans present; the age and species of fish present; and the areas of infection. The parasitic copepod family Lernaeidae causes dermatosis, a condition that can have major harmful effects on its fish hosts.31
Lernaea or Anchor Worm parasites cause it, with L. cyprinacea being the most common. The mature female is a worm-like fish parasite that burrows into the fish’s flesh and keeps its egg casings jutting out of the fish’s body. Males attack the fish and are not adapted to parasitism. Parasites feed on blood and epithelium and are discovered attached to gill filaments. They may later be discovered on the fins and body. The infection causes decreased breathing, epithelial enlargement, anemia, slowed growth, agitation, and, eventually, death. Secondary infection, particularly fungus, makes the fish vulnerable.33
Epidemiology of Copepods
The revision of the family Lernaeidae has identified 12 legitimate species in this genus. The genus Lernaeenicus was recently described by Gopalakrishnan et al.34 Infections with ergasilids are usually modest in African lakes and rivers. During the dry season, restricted fish may become infected in environments such as regressing pools in river beds. It has been reported that significant infections play a role in the mass mortality of this fish that occurs at times in the lakes.22
When their hosts are translocated, the exacting, nutritional, and environmental needs of the free-living stages appear to limit Ergasilid dispersal. This is emphasized by the fact that only a few species of Ergasilus and related copepod genera have ever established themselves in the pond.34
Prevention and Treatment of Copepods Disease
In most circumstances, a comprehensive fish health management program should be founded on the concepts of water quality, nutrition, sanitation, and quarantine.15 Disease prevention is always more satisfying than disease treatment. As a result, avoid acquiring or adding fish to a newly formed tank that demonstrates strange swimming behavior. Signs of fin and body injury.14
Ergasilus can be treated with a 3% salt dip followed by a 0.2% extended dip for three-weeks (Drug Authority and Control Agency (DACA)).35 Lernaea is exceedingly difficult to control be cause therapy primarily affects free-living larvae.16
BRANCHIURA
Branchiura consists of approximately 175 species, divided into four genera and put into a single family, the Argulidae. The body of an Argulus (fish lice) consists of five limb-bearing segments, a trunk separated into a thoracic region containing four pairs of powerful swimming legs, and a short abdomen with a single bilobed unit caudal fin.9
The genus Argulus contains the most common Branchiura members. Many studies have found that parasites and other illnesses are transmitted through freshwater fish all over the world. Over 170 Argulus species have been identified in freshwater and marine fish.36 Dorso-ventral flattening and dorsally covered by a rounded or horseshoe-shaped carapace for attachment, ventral head appendages are created. Argulus species are causing significant losses in fish stocks in various nations. These species have been documented from marine fish farming operations in nations such as Chile, Canada, and Norway. Furthermore, they can cause mortality in farmed fish stocks.37,38 As a result, the current study was designed to look at Argulus species, which are common parasite diseases in freshwater fish.36
Characteristics of Branchiura
Argulus fish lice are frequent parasites seen in fish. It is a big ectoparasite that may migrate across the fish’s body surface. Argulus species (Family: Argulidae) are frequent freshwater fish parasites.39 Many studies have found that parasites and other illnesses are transmitted through freshwater fish all over the world. Argulus species are depleting fish stocks in several countries. These species have been documented from marine fish farming operations in Chile, Canada, and Norway, among other places. Furthermore, they can be lethal in farmed fish stocks.37,44 As a result, the current study was designed to look at Argulus species, which are common parasite infections in freshwater fishes.36
Life-cycle Branchiura
Males in the genus Argulus and Dolops deposit spermatophore onto females, and once the eggs are fertilized, the females leave the host organism to lay their eggs on surfaces such as plants, rocks, and so forth.40 Males and females can both live in the wild for up to 15-days. Males transmit sperm directly to females via a variety of modified structures on the third and fourth thoracic legs in most brachyurans.41 Argulus species have a direct life cycle using fish as hosts. In other words, Argulus species live for the majority of their life cycle on fish species as hosts.42
Argulus is well-known, but nothing is known about the other genera or marine species. After consuming a meal, a mature female Argulus will leave its host and begin to lay eggs in rows on any hard, submerged surface in freshwater species. At any one time, up to 1200 eggs are laid and glued to the substrate. The abandoned eggs hatch between 12 and 80-days, depending on the species, although development time is also highly dependent on temperature. Eggs in a string hatch in about a week. These eggs hatch into free-swimming larvae with setose swimming antennae and mandibles, as well as rudiments of the maxillae and the first two pairs of swimming legs. These larvae serve as a dispersion phase before molting into the second stage, in which powerful claws replace the setae on the antenna and the mandible’s setose palp is destroyed.43 The first larval stage lasts around six-days, with molts occurring at regular intervals until maturity. Branchiurans appear to leave the host and then find a new host at intervals throughout development. Except for the maxillae, which undergo a profound metamorphosis around the fifth larval stage, changing from a long limb-bearing to a powerful distal claw into a short but powerful circular sucker, changes during the larval phase are gradual.44
Pathogenesis Branchiura
Argulus or fish? Lice is a widespread Branchiura parasite found in fish and has also been found in frog tadpoles. It is a big ectoparasite that may migrate across the body surface of the fish. All life stages of both sexes are parasitic.10 It clings to its host, generally a fish, with suction cups, pierces the skin with its sharp style, and feeds on blood. It could live in the gills.45
A severe infestation produces skin inflammation, open hemorrhaging wounds, increased mucus production, scale loss, and fin erosion. The wounds are frequently infected with bacteria and fungi, which further damage the skin layer. Anemic fish might develop during feeding; the louse injects digestive enzymes to start breaking down host tissues before absorption. Infested fish may exhibit behavioral indications such as erratic swimming and rubbing up against aquarium walls, as well as a decrease in appetite and reduced growth.46 Stress and mortality sites become hemorrhagically ulcerated as a result of injury and infection. When the skin, fins, and gills get contaminated, mucus is secreted.45
The distinction between Dolops spp. and Argulus sp. is that Argulus sp. possesses suction discs, which are modified first maxillae, whereas Dolops spp. contains hooks and accompanying spines. They either pierce the host’s skin and suck blood and other internal fluids, or they feed on mucus and skin sloughed off by the host. Fish lice and other attached ectoparasites cause epithelium damage and stress responses, which can lead to secondary consequences.47
Epidemiology Branchiura
Argulus foliaceus (Crustacea, Branchiura) is a branchiuran parasite found on a broad variety of fish species, as well as amphibian tadpoles. Common fish live in marine, brackish, and freshwater settings.48
In Africa, 29 indigenous species of fish from various families can be found. Argulus africanus and Dolops ranarum are opportunists that can be found in a variety of fish throughout Africa’s major systems. D. ranarum is the only ubiquitous argulid that is possibly pathogenic to farmed fish. The late Albertianus of Lakes Albert and Kyoga, as well as the Victoria Nile, are infected with D. ranarum. Japonicus is exposed to a major pest of farmed carp that was previously rarely observed on pond-reared carp.22
Prevention and Treatment of Branchiura
Argulus foliaceus will readily deposit its eggs on hard items such as wooden boards, which can be removed from the water to lower the egg load in the fishery.49 A short bath in a sodium chloride solution can lower the parasite burden on a fish, but this treatment must be done carefully because too short a bath or to dilute a solution is ineffective, while too long or too intense a bath can injure the fish.50
ISOPOD
In tropical and subtropical seas, isopods are regularly encountered on teleosts clinging to the body surface, in the mouth, or on the gills. The body is divided into three sections: the head, which is unsegmented and has two pairs of antennae, two pairs of variable-sized eyes, and a mouth. The vast majority of them are ectoparasites.11
Cymothoids, epicaridians, and gnathiids are the three main groups. Cymothoids are fish parasites that exist in both juvenile and adult forms. Epicaridians are crustacean parasites as adults and immatures. Gnathiids are fish larval parasites that are free-living and non-feeding adults. Cymothoids and epicaridians appear to have a close genetic relationship, although gnathiids appear to have developed from separate isopod lines.41
Characteristics of Isopods
The bulk of crustaceans are aquatic, and the isopods are one of the few groups whose members now dwell on land.51 One of the most distinguishing features of parasitic isopods is that they are frequently maritime and live in warmer oceans.12 These are the isopods that are regularly encountered on teleosts, fish subclasses, and in tropical and subtropical waters, clinging to the body surface, in the mouth, or on the gills.11,52 Male cymothoids are often narrower than females, and the ratio of length to breadth has been employed as a measure of femininity.53,54
Life Cycle of Isopods
Gravid females deposit their eggs in a brood pouch, or marsupium, produced by their ventral oostegites. Cymothoidae have only one host (the Holoxenic cycle), attach early in life to fish, and go through a male stage (Cymothoids are protandrous hermaphrodites).12
The first male parasitizing a fish transforms into a female. Males who connect to the same fish stay males. It appears likely that the female releases a pheromone or neurohormone that limits further growth of males before becoming females or that the presence of a mature female stops male-stage specimens from further development.41
Pathogenesis Isopod
Isopod parasites have gotten a lot of attention in the scientific community since they generate a lot of problems for fisheries. Cymothoid spp. cause severe issues in captive or caged fish. They inflict harm similar to other copepods, but the most significant effect of isopod infection is the loss of host tissue caused by the parasite’s body pressure. On the fish, there is pressure necrosis at the site of the dwelling region. The degree of harm to fish varies according to the site of attachment and the isopod-to-host ratio. A lesion to the integument induces hyperplasia, or desquamation, followed by cutaneous inflammation and necrosis.13
Secondary infections arise as cymothoids with their pereopods and mouthparts penetrate the skin, and the tissue-dwelling forms keep a little hole to the outside. They may provide entrance routes for microbial illnesses through the wounds they generate.12
Epidemiology Isopod
Cymothiod isopods (Lironeca spp.) have been found in African freshwater fish (presumably as marine remnants) in the Congo basin (in cichlids, clupeids, and citharinids). Cymothiod isopods (Lironeca spp.) have been found in African freshwater fish (presumably as marine relics) in the Congo basin (in cichlids, clupeids, and citharinids). Parasitic isopods are commonly found in warmer oceans, and infections have been documented in euryhaline fish in estuaries.22
The prevalence, intensity, host/site specificity, and seasonal fluctuation of parasitic isopods infecting clupeidaen fish on the Malabar coast (India) were studied. Even though the incidence of many parasites varies noticeably from season to season, research on the seasonality of parasitic occurrence is relatively limited.29
Prevention and Treatment of Isopod
Because infection by the planktonic phase is a common occurrence, no unique control or therapeutic measures against isopods have been adopted. Quarantine is the first line of defense (with close observation of new fish), mechanical removal with forceps is sometimes possible, osmotic shock (freshwater or saltwater dips) is sometimes effective, water treatments with organophosphates are sometimes warranted, and diflubenzuron may also be effective. When contemplating isopod infection treatments, keep in mind that some isopods can detach from and live apart from their hosts for extended periods of time. Isopods can be mechanically removed from aquarium fish and preserved in 10% formalin or 70% ethanol and stored in 70% ethanol (151 proof rum will do) or 40% isopropanol (rubbing alcohol). When dealing with gnathiid diseases, keep in mind that only the larvae are parasitic and that adults can be found on the substrate. Thus, treating diseased fish without correcting their habitat may only provide short-term relief. It is particularly critical to recognize the risk of secondary infections associated with severe isopod infections.16,54
CONCLUSION AND RECOMMENDATIONS
External parasitic infestations by crustaceans are a significant concern for both wild and cultivated fish populations. Copepods (Ergasilidae and Lernaeidae), Branchiura (Argulidae), and Isopods are among the most common parasitic crustaceans affecting fish. These parasites can lead to gill obstruction, mechanical damage, stress, secondary infections, and other health issues. Effective prevention and management strategies, including quarantine, water quality management, sanitation, mechanical removal, freshwater or saltwater dips, and chemical treatments, can help reduce the impact of these parasites on fish health and productivity. As the understanding of host-parasite interactions continues to evolve, the development of innovative control methods remains essential to safeguarding fish populations and ensuring their long-term sustainability.
Hence, the following recommendations are rewarded based on the above conclusion:
• Veterinarians require greater information and knowledge regarding crab parasites.
• Caution should be exercised while introducing new fish into the population.
• Studies on the life cycle and physiology of crustacean parasites to determine the best chemical (drug) at the right moment are recommended.
• Further research on the epidemiology and ecology of external parasites, particularly crustaceans, should be encouraged.
DATA AVAILABILITY
All the datasets generated or analysed during this study are included in this manuscript.
AUTHORS’ CONTRIBUTIONS
IAK and HFG Study conception, data collection, designed the study, and drafted and wrote the manuscript; ADA, SK and GD edited the manuscript, revised it, and searched the references. All authors have approved the submission of the final manuscript.
FUNDING STATEMENT
The current study was conducted without the support of funding sources.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






