INTRODUCTION
Positron emission tomography/computed tomography (PET/CT) following administration of radiotracer 18F fluorodeoxyglucose (18F-FDG) is currently considered standard of practice for the management of lymphoma.1 The diagnostic sensitivity and specificity of PET/CT in the initial staging of intermediate or high grade non-Hodgkin lymphoma has been found to approach 100%.2 It provides metabolic information by way of a semi-quantitative index, the standardized uptake value (SUV) which has been shown to be useful for determining disease prognosis and treatment response.3,4
However, PET/CT is expensive, time-consuming, requires exposure to ionizing radiation and is not widely available as it involves the use of a cyclotron.5 In addition, FDG is not entirely cancer-specific; brown fat, muscle tissue and other factors such as infection, inflammation and granulomatous disease can also demonstrate increased FDG uptake. On the other hand, magnetic resonance imaging (MRI) although expensive, does not involve exposure to ionizing radiation, is more widely available and provides excellent soft tissue contrast. In addition, with rapidly progressive MRI techniques such as echo planar imaging and parallel imaging techniques, whole body MRI examinations can be performed within an acceptable time frame.
The evaluation of nodal involvement by CT and conventional MRI are dependent on morphology as determined by the response evaluation criteria in solid tumors (RECIST) and the World Health Organization (WHO).6,7 However, large nodes may be reactive and small nodes can still harbor foci of disease.
Diffusion weighted imaging (DWI) is a non-invasive technique used for imaging molecular movement or diffusion that is responsive to changes in microstructure of biological tissues. Apparent diffusion coefficient (ADC) is a quantitative index of DWI which can be measured by fitting the MRI signal intensity attained from images with different diffusion-weighting to a monoexponential decay model. It is a relatively simple method and can be attained with as few as two sets of DWI data. DWI with ADC can provide useful physiological and functional information with regards to the characterization of lymph nodes.8,9 Due to high cellularity and high nuclear-to-cytoplasmic ratio, lymphoma results in reduced extra-and intracellular dimensions which leads to high signal intensity on DWI and reduced ADC.
Post-treatment, organizing granulation tissue and fibrotic changes are paucicellular. As a result, DWI should be an effective imaging modality to discriminate between viable lymphoma and treated disease. Several studies in varying cancers have suggested that DWI may potentially be able to discriminate between viable residual tumor and non-neoplastic treatment changes post-therapy.10,11,12,13,14
PET/CT will be used as a reference standard in the evaluation of the value of whole body DWI (WB-DWI) in the initial staging and treatment response in patients with lymphoma.
METHODS
Patients
From January 2012 to January 2015, nine patients (6 men and 3 women; mean age, 53 years) with newly diagnosed lymphoma and a pre-treatment PET/CT were prospectively recruited in this single centre study.
The study was approved by the local institutional review board and written informed consent was obtained from all study participants. The study was performed in accordance with the ethical standards laid down in the 1964, declaration of Helsinki and all subsequent revisions. Inclusion criteria were as follows: Age between 21 to 80 years, histological diagnosis of lymphoma, scheduled for first line chemotherapy and nodal or extra nodal disease measuring at least two centimeters in size. Exclusion criteria were 1) General contraindications to MRI (such as ferromagnetic implants, pacemakers and claustrophobia), 2) Pregnant or lactating patients, 3) Non-FDG avid or structurally non-measurable lesions in the pre-treatment PET/CT study.
A detailed medical history and physical examination was obtained for all patients along with standard laboratory tests. Symptoms such as unexplained weight loss, fever and night sweats, co-morbidities, and ECOG (Eastern Cooperative Oncology Group) score were recorded and patients were examined for palpable nodes and presence of hepatosplenomegaly. Seven of the nine patients were diagnosed with diffuse large B-cell lymphoma (DLBCL) and the remaining two had peripheral T-cell lymphoma (PTCL). Ann Arbor staging at time of diagnosis was stage IE in one, II in four and one each in IIE, III, IIIE and IV.
All pre-treatment whole body MRI studies were performed from one to 34 days post PET/CT. All patients received standard first-line chemotherapy after the baseline MRI studies. Five patients received R-CHOP [Rituximab, Cyclophosphamide, Hydroxydaunorucibin (Doxorubicin), Oncovin (Vincristine) and Prednisolone], two received MR-CHOP (Methotrexate, CHOP) and remaining two received CHOPE (CHOP, Etoposide).
Imaging Time-points and Techniques
All patients were followed clinically throughout the study and evaluated with both MRI and PET/CT before treatment initiation and at four weeks after completion of chemotherapy.
MRI was performed using a 1.5-T MR system (Siemens, Aera). Patients were scanned from vertex to mid-thigh using a 20-channel head and neck coil, 32-channel spine coil and two 18-channel body coils. The MRI protocol included axial HASTE TIRM, axial FL2D in and out phase, axial DWI b=50, 800, coronal T1 and coronal TIRM. All MR images were reviewed on a picture archiving and communication system (PACS) workstation.
For PET/CT, patients were scanned from vertex to mid-thigh with an integrated PET/CT that was acquired approximately 60 mins after intravenous injection of a weight-related dose of 18F-FDG tracer under fasting conditions (6 hours). All PET/CT data was evaluated on a computer display in three orthogonal planes (axial, coronal and sagittal).
Image Interpretation
The presence or absence of nodal involvement in each of twenty regions (Table 1) was independently reviewed by two radiologists blinded to the PET/CT results. Any initial disagreements between the two observers about the imaging findings were resolved in consensus. The PET/CT images were reviewed by a single nuclear medicine physician. The presence or absence of extra nodal involvement was also made in the following sites: Lung, liver, spleen, bone marrow, stomach/other gastrointestinal sites, kidneys and others. A total of 27 regions were assessed for each patient with each imaging modality.
| Table 1: Twenty Regions of Nodal Involvement that were Independently Assessed by Two Radiologists on WB-DWI and a Single Nuclear Medicine Physician on PET/CT. |
| No. |
Nodal Region |
| 1 |
Waldeyer’s ring and retropharyngeal |
| 2 |
Right cervical/supraclavicular/occipital/pre-auricular |
| 3 |
Left cervical/supraclavicular/occipital/pre-auricular |
| 4 |
Right axillary/Pectoral |
| 5 |
Left axillary/Pectoral |
| 6 |
Right internal mammary/diaphragmatic |
| 7 |
Left internal mammary/diaphragmatic |
| 8 |
Anterior mediastinal |
| 9 |
Right paratracheal |
| 10 |
Left paratracheal |
| 11 |
Subcarinal/posterior Mediastinal |
| 12 |
Right hilar |
| 13 |
Left hilar |
| 14 |
Retroperitoneal (paraaortic/paracaval/inter-aorta-caval/peripancreatic) |
| 15 |
Abdominal (Periportal/Perisplenic/Periceliac/Perisuperior mesenteric artery/Periinferior mesenteric artery) |
| 16 |
Mesenteric |
| 17 |
Right Iliac |
| 18 |
Left Iliac |
| 19 |
Right Inguinal/femoral |
| 20 |
Left Inguinal/femoral |
All DWI images were qualitatively interpreted in association with ADC maps; hyperintensity on DWI with a corresponding ADC signal which was hypointense to muscle indicated restricted diffusion. For PET/CT, nodal and extra nodal involvement was diagnosed when any area of abnormal focal increase in tracer uptake was visually evident. For quantitative evaluation of WB-DWI, the mean ADC value of the largest involved node in a region was measured directly on ADC maps; the region of interest (ROI) was manually drawn avoiding any areas of necrosis. The SUV of the largest involved node in a region was recorded for PET/CT.
During assessment of both imaging modalities post-completion of chemotherapy, an evaluation of response to treatment was made and categorized into progressive disease, stable disease, partial response and complete response in comparison to the pre-treatment study. New and/or larger foci of disease involvement was interpreted as progressive disease, no significant change in size and distribution of disease was regarded as stable disease, decrease in size and/or number of lesions was interpreted as partial response and no abnormal lesions in the follow-up study was deemed complete response.
Statistical Analysis
Sensitivity, specificity and accuracy of PET/CT and WB-DWI were compared to evaluate the difference between modalities both in the pre-treatment studies and post-completion of chemotherapy. A correlation was also made between SUV and ADC values to determine a possible relationship between the values obtained. This could only be applied to lesions that were found to be positive on both PET/CT and WB-DWI.
RESULTS
Baseline Staging
In the nine patients, PET/CT defined a total of 72 out of 243 (29.6%) regions as positive for disease involvement with 171 out of 243 (70.4%) as being negative.
Compared to PET/CT, the sensitivity and specificity of WB-DWI was 60.3% (38/63) and 100% (116/116) respectively, for nodal disease and 75.0% (6/8) and 92.7% (51/55) respectively for extra-nodal disease. Overall sensitivity, specificity and accuracy of WB-DWI compared to PET/CT was 62.0% (44/71), 97.7% (167/171) and 86.8% (211/243).
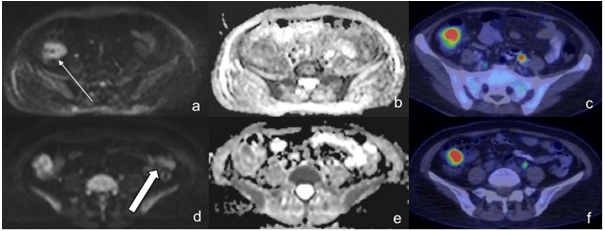
In a third (3 out of 9) of the patients, PET/CT was more sensitive in detecting disease in Waldeyer’s ring (Figure 1). In two patients, PET/CT was considerably more sensitive with WB-DWI missing 50% (10/20) and 35% (7/20) of nodal regions. This was primarily a result of small nodes which demonstrated uptake on PET/CT but were not identified or did not show restricted diffusion on WB-DWI (Figures 2-5).
Figure 1: In 3 out of 9 Patients, PET/CT Demonstrated an Asymmetrical Increased Uptake (a,d,g) in Waldeyer’s Ring which was Interpreted as Lymphomatous Involvement. However, Corresponding DWI (b,e,h) and ADC (c,f,i) Failed to Show Restricted Diffusion or Asymmetry which may have Led the Readers to Suspect Lymphomatous Involvement.
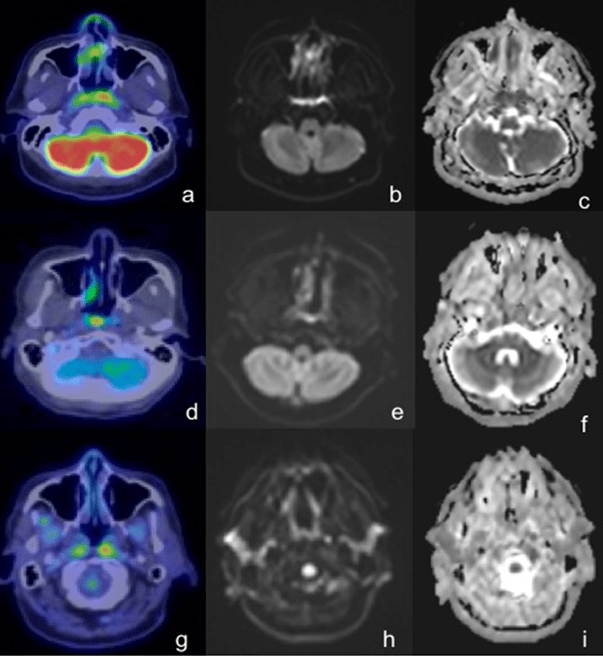
Figure 2: 57-Year-Old Male with Right-Sided Cervical Nodal Involvement was Detected on both Modalities (thin arrow). However, Only Left Cervical Adenopathy was Identified on PET/CT (a, Thick Arrow) with No Evidence of Restricted Diffusion on WB-DWI (b,c).
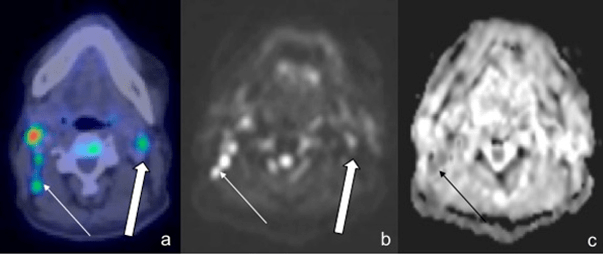
Figure 3: A 57-Year-Old Male with DLBCL. A Small Right Axillary Node with Increased Uptake was detected on PET/CT (a, thin arrow). However, it was not seen on either DWI or ADC images. Note increased uptake on PET/CT in the left upper rib with corresponding restricted diffusion on WB-DWI (b,c, thick arrow)
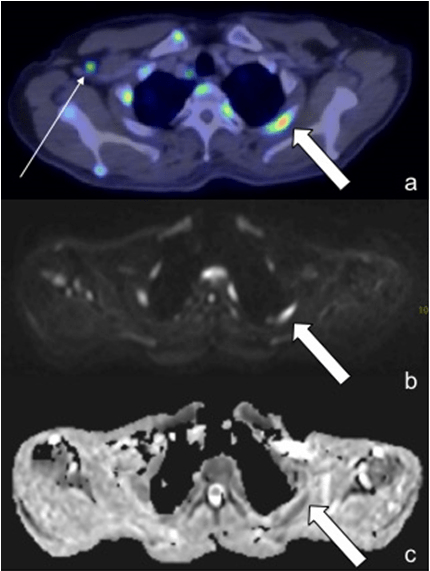
Figure 4: A 65-Year-Old Female with DLBCL. PET/CT Demonstrated Increased Uptake in the Mediastinal Nodes (a, Thin Arrows), However, these were not even Visualized on WBDWI (b,c). In Contrast, the Right Axillary Node (Thick Arrows) were Positive on both Modalities (a-c).
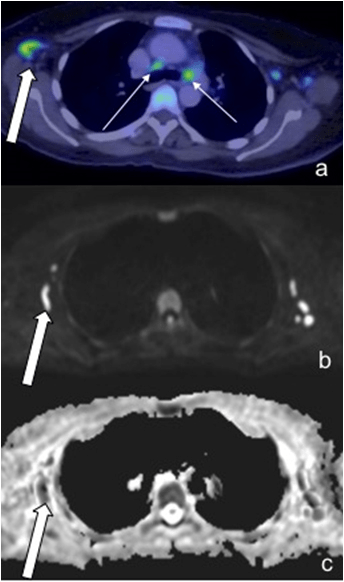
Figure 5: A 57-Year-Old Male Patient Demonstrated Large Pulmonary Lesions with Intense FDG Uptake (a, Thick Arrow), However, these were Barely Detectable on DWI and Misinterpreted as Artefact on ADC (c, Dashed Arrow). Small Hilar Nodes (a, Thin Arrow) were not seen on WBDWI. Note Increased FDG Uptake in the Vertebra, Sternum and Ribs with Corresponding Restricted Diffusion.
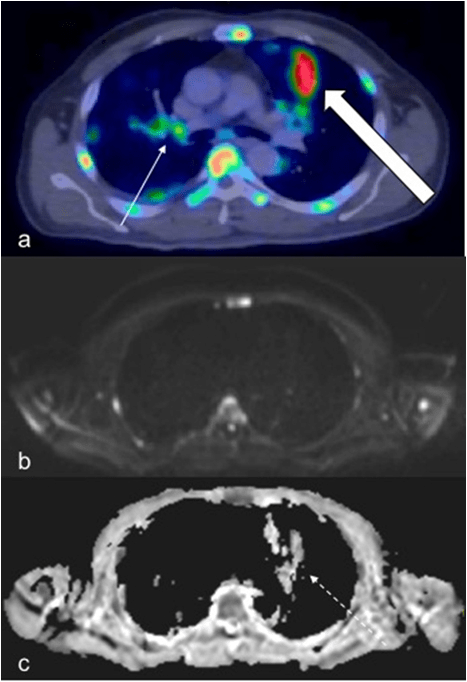
One of the patient also exhibited florid pulmonary lesions that were easily seen on PET/CT but did not demonstrate significant signal change on DWI and were misinterpreted as arte fact on ADC (Figure 5). In addition, two extra-nodal regions in the same patient were found to be false positive on WB-DWI, this was due to: 1). Misinterpretation of the spleen as involved as it was hypointense relative to the liver on ADC, PET/CT however, did not reveal increased uptake (Figure 6), 2). Misinterpretation of the stomach as being involved (Figure 7), this was primarily the result of superior spatial resolution of CT compared to MRI as adjacent nodal involvement was misread as gastric involvement on MRI.
Figure 6: In a 57-Year-Old Male Patient, the Spleen was Misinterpreted as having Lymphomatous Involvement as it was Relatively Hypointense to the Liver on ADC (a, Thin Arrow). However, Findings on PET/CT (b) Demonstrate no Increased Splenic Uptake.
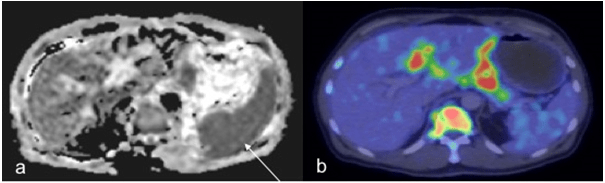
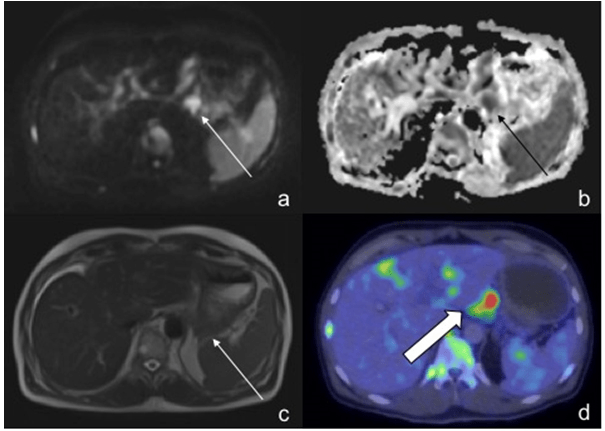
Figure 7: In a 57-Year-Old Male Patient, the Gastric Fundus was Misinterpreted as Being Involved (Thin Arrow) on DWI (a) and ADC (b) as well as on T2W (c) imaging. However, PET/CT was Able to demonstrate a Nodalfocus Separate to that of the Stomach (d, Thick Arrow) illustrating the superior spatial resolution of CT over MRI.
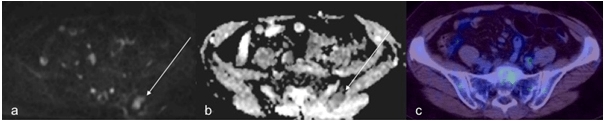
WB-DWI was generally effective in the determination of bone marrow infiltration where it was positive on PET/CT (Figure 5). However, there was a false positive result wherein a focus of restricted diffusion was noted in the left posterior ilium that was found to be negative on PET/CT (Figure 8).
Figure 8: A 65-Year-Old Female Patient with DLBCL, a Focus of Restricted Diffusion (a,b) in the Left Posterior Ilium (thin arrow) was found to be False Positive with no Increased Uptake Detected on PET/CT (c).
Treatment Response
One patient withdrew from the study after the baseline studies. As a result, treatment response could only be assessed for eight patients. All except one patient were assessed as having complete response on PET/CT with the remaining patient deemed as having a partial response with a single extra-nodal region in the caecum demonstrating persistent increased uptake. All except one patient were also assessed as having complete response on WB-DWI with the remaining patient deemed as having a partial response with two false positive nodal regions.
As there was near complete response, true positive findings were not found on WB-DWI compared to PET/CT. Therefore, sensitivity could not be assessed. Compared to PET/CT, the specificity of WB-DWI was 98.8% (158/160) for nodal disease and 100% (55/55) for extra-nodal disease. Overall specificity and accuracy of WB-DWI when compared to PET/CT was 99.1% (213/215) and 98.6% (213/216).
The false negative finding on WB-DWI involved a caecal lesion; it was picked up on pre-treatment WB-DWI as it was distinctively hyperintense whilst the remaining bowel was hypointense. However, on post-treatment images, segments with scattered hyperintensity were also seen in the remaining bowel which made the caecal lesion less conspicuous resulting in it being missed. In contrast, the caecal lesion remained clearly and visually perceptible on PET/CT in both the pre- and post-treatment studies (Figure 9).
Figure 9: A 65-Year-Old Female Patient with DLBCL, in the Pre-Treatment DWI (a), the Caecal Lesion was Distinctively Hyperintense (Thin Arrow) in Comparison to the Remaining Hypointense Bowel. However, on Post-treatment Studies DWI (d), this was Less Apparent as Other Bowel Loops also Showed Hyperintensity, such as in the Descending Colon (Thick Arrow). ADC Maps (b, e) were not Particularly Useful. In Contrast, the Caecal Lesion was Distinctive in both Pre- and Post-treatment PET/CT Studies (c, f).
Correlation between SUV and ADC Values
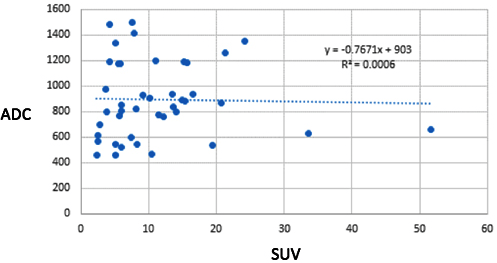
Involved nodes showed increased uptake on PET/CT, high signal intensity on DWI and low signal intensity on ADC maps (Figure 10). The SUV of the largest node in an involved region was compared to the mean ADC value of the largest node in an involved region. There was a total of 43 lesions that were found to be positive both on PET/CT and WB-DWI. One would expect an inverse correlation between the ADC value and the SUV. However, no significant inverse correlation was seen in our study (r=-0.024, p=0.88) (Figure 11).
Figure 10: In a 61-Year-Old Male Patient with DLBCL, a Large Right Cervical Node Demonstrated Typical Hyperintensity on DWI (a) with Corresponding Hypointensity on ADC (b) and Intense Uptake on PET/CT (c).
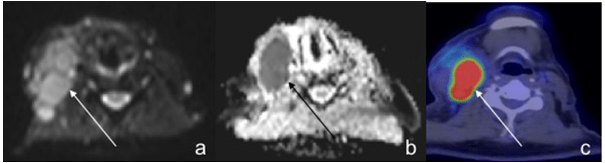
Figure 11: Plot of ADC against SUV of all Nodal and Extra-Nodal Regions that were found to be Positive on Both PET/CT and WB-DWI. Although an Inverse Correlation was Expected, it was not shown in this Study.
DISCUSSION
DWI was initially applied in the brain and found to be exceptionally useful for evaluation of restricted diffusion in the diagnosis of acute infarction. However, in recent years, there has been progressively increased use of this technique in the rest of the body.
Lymphomatous tissue, due to increased cellularity and reduced extracellular space results in restricted diffusion (hyperintensity on DWI and hypointensity on ADC). Whole body imaging is desirable in the assessment of hematological malignancies such as lymphoma. Hence, WB-DWI is gaining increased attention as an alternative to PET/CT in the staging and treatment of lymphoma as it can assess the entire body, is radiation-free, more accessible and does not require use of a contrast agent. There are however deficiencies with the use of WB-DWI relative to PET/CT, some of which were illustrated in this study and will be discussed further below.
Waldeyer’s ring may exhibit normal high signal on DWI due to relatively low diffusivity and long T2 value.15 Hence, detection of abnormality in this area on WB-DWI can be difficult as was seen in a third of patients in this study. The asymmetrical increased uptake on PET/CT was not depicted on WB-DWI which may otherwise have led the readers to suspect lymphomatous involvement; this was likely secondary to the high baseline signal on DWI which obscured overlying pathological involvement.
The use of DWI in the chest cavity is limited secondary to artefacts caused by respiratory or cardiac motion. This is likely the cause of the false negative WB-DWI pulmonary findings in one patient where florid lung lesions were depicted on PET/CT. Use of cardiac or respiratory triggering WB-DWI may help to overcome this.16
Liver or splenic involvement may be misinterpreted on WB-DWI with the latter occurring in one patient. This may be due to iron content which has been shown to affect DWI visibility and ADC quantification in normal hepatic parenchyma, normal DWI liver visibility is also gender-and age-dependent being superior in women before the age of fifty.17
Several artefacts are encountered while assessing the bowel in DWI. Depending on luminal contents, T2-shine through effect will result in hyperintense bowel loops. The true presence of restricted diffusion can then be determined by evaluation of corresponding ADC maps.18 However, due to inherent heterogeneity of bowel contents, it is frequently challenging to differentiate true restricted diffusion among T2-shine through effect. In addition, bowel peristalsis resulting in motion artefact and inferior spatial resolution of MRI contribute to the challenges in diagnosis of bowel lesions in WB-DWI.
Majority of false negative nodes on WB-MRI were small nodes that were either not even depicted on WB-DWI or did not demonstrate convincing restricted diffusion. This is likely a combination of inherent increased sensitivity of PET imaging which is in the picomolar range19 and relative increased susceptibility to motion artefact on MRI.20
A further shortcoming of WB-DWI is in the assessment of bone marrow. This is particularly important during skeletal maturation. During maturation, hematopoietic marrow is gradually converted to fatty marrow. On DWI, fatty marrow is hypointense secondary to fat suppression whereas red marrow is hyperintense due to relatively higher cellularity. Hence, residual or reactivated islands of red marrow, may conceivably be misinterpreted as lymphomatous involvement in WB-DWI.15 In contrast, Albano et al21 conducted a study comparing whole-body MRI, FDG-PET/CT and bone marrow biopsy and found that both MRI and PET/CT were valuable tools for the assessment of bone marrow involvement.
Our results for baseline staging demonstrate that the nodal sensitivity and specificity of WB-DWI compared to PET/CT was 60% and 100% respectively and extra-nodal sensitivity and specificity was 75% and 93% respectively. Overall, WB-DWI showed 62% sensitivity and 98% specificity as compared to PET/CT. Results for treatment response were superior with 99-100% specificity and accuracy for nodal and extra-nodal disease. Compared to PET/CT, the reported sensitivity and specificity for assessment of lymphoma with WB-DWI is 89-100% and 97-100% respectively.20,22,23,24 Our results for specificity of WB-DWI are comparable to that of PET/CT (93-100%) and are in line with previous observations. In contrast, our results for sensitivity were inferior to that seen in previous studies; this may be secondary to our small sample size. However, the reduced sensitivity of WB-DWI compared to PET/CT is likely due to inherent increased sensitivity of PET over MRI with many of our false negative results due to small nodes. They demonstrated uptake on PET/CT but were either not seen on or did not show restricted diffusion on WB-DWI.
Our finding of inferiority of WB-MRI in comparison to PET/CT in staging assessment contrasts with several studies16,24,25,26,27 that support DWI as an alternative imaging modality for the initial staging of lymphoma. Although our sample size is small, important differences have been illustrated between the imaging modalities that demonstrate the strength of PET/CT over WB-DWI which account for superiority of PET/CT in baseline staging of lymphoma. This is supported in part by a study conducted by Mayerhoefer et al20 that found that DWI was slightly inferior to PET/CT with regards to pre-therapeutic regional assessment and staging in patients with FDG-avid lymphoma. However, they found that this was not the case in patients with lymphoma subtypes that showed variable FDG avidity. Another study22 found that in a minority of their patients with newly diagnosed lymphoma, whole-body DWI led to clinically important over-staging relative to the results of PET/CT thereby concluding that PET/CT remain the reference standard for lymphoma staging lending support to our conclusion.
There were several limitations to this study, the first is having a small number of patients. Despite this drawback, important false positive and false negative findings with respect to WB-DWI were illustrated. We did not perform cardiac or respiratory triggering for WB-DWI resulting in motion artefact limiting evaluation of the mediastinum, lung and left hepatic lobe.28 The mean ADC value and SUV may have been derived from different nodes within an involved nodal region on WB-DWI and PET/CT respectively; this might have contributed to the poor inverse correlation seen in this study. Lastly, although PET/CT is currently considered the standard of practice in management of lymphoma, using it as a reference standard obscures possible inherent limitations of this modality.
CONCLUSION
In conclusion, WB-DWI may be a viable alternative to PET/CT in the treatment response of lymphoma, this is of particular importance in younger patients to lower radiation burden and future risk of radiation-induced malignancies. In terms of staging however, PET/CT is probably still the superior modality, but if MRI is used instead, care should be taken to understand the limitations of this modality such that has been illustrated above.
FUNDING
This study was funded by Cancer Research Grant from Singapore Cancer Society.
ACKNOWLEDGEMENT
Research administration support from Ms. Lim Tze Wei.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
















