INTRODUCTION
The equine respiratory tract is a highly specialized system facilitating the movement of large volumes of air in and out of the lungs each minute. Any structural or functional disorder associated with the lungs can lead to exercise intolerance and poor performance.1
Respiratory disease was identified in 40% to 42% of racehorses presented for poor performance evaluation. Of the horses identified with respiratory dysfunction, dorsal displacement of the soft palate was the most commonly identified respiratory disorder, affecting 29% to 35% of racehorses. Laryngeal hemiplegia was identified in 17% to 24% of the racehorses. Pharyngeal collapse was identified in 27% in one study and in only 1.5% in another study. Retrospective analysis of sport horses presented for poor performance also identified respiratory disease disorders in 54 of 77 (70%) sport horses. Laryngeal hemiplegia was identified in 40% of these horses. Dorsal displacement was identified in only 11%, and pharyngeal collapse was identified in 30%. Results of the retrospective analysis of sport horses suggest that respiratory compromise may be over-represented in this population. Additionally, laryngeal hemiparesis and pharyngeal collapse represent most of the respiratory problems in sport horses.2
Respiratory diseases are the result of exposure to dust and other airborne irritants. Besides; bacteria, mold and ammonia levels. Excess nasal discharge, coughing, sneezing and mucus secretion are all signs that a horse may be suffering from respiratory problems.3
Endoscopy is become routine over the last decades because it an excellent tool for diagnosis of the respiratory disorders. It facilitates visualization of inaccessible areas of the pharynx, larynx, guttural pouches and trachea.3,4,5
Also, the upper respiratory endoscopy is used for diagnosing many of the respiratory disorders namely dorsal displacement of soft palate (DDSP), epiglottic entrapment, roaring, infections and taking samples for bacterial cultures and histopathological examinations. Biopsy samples are assumed for diagnosing many types of tumors, inflammatory processes, as well as removal of foreign objects that have been inhaled into the respiratory system.6
The aim of the current study is directed toward the endoscopic examination of upper respiratory tract throughout highlighting and clarification of the disease conditions that are met with the respiratory distressed horses and donkeys, as these diseases or abnormalities negatively affect work and sports ability. Consequently, the study will assist to pave away for the hopeful and future treatment of such disease evidences.
MATERIAL AND METHODS
The present work employed the endoscopic examination of the upper respiratory tract of 45 stallions, 30 mares, 10 foals and 38 donkeys suffering from upper respiratory tract distress. These animals belonged to various age groups and weights (Table 1). The study was conducted following the receipt of the approval from the Animal Ethics Committee of Faculty of Veterinary Medicine, Cairo University, Giza, Giza Governorate, Egypt.
| Table 1: Demonstrating the Number, Age and Body Weight of Animals of Study. |
| Animal |
Number |
Age/year |
Body weight/kg |
| Normal |
Diseased |
| Foals |
2 |
8 |
6 months-3 years |
100 – 250 |
| Stallions |
20 |
25 |
11-17 |
450 – 600 |
| Mares |
21 |
9 |
| Donkeys |
24 |
14 |
3-6 |
150 – 250 |
| Total |
67 |
56 |
Prior to the endoscopic examination using equine flexible video-endoscope (Eickemyer 3000 Vmec, Germany), each animal was subjected to fasting for 4-6 hours and withheld from water consumption for 4 hours. Each animal twitched and stood in the stanchion. Vicious animals were injected with Xylazine HCl (Xyla-Ject 2%®, Adwia Co. SAE- Egypt; 1.1 mg/kg. b. wt) used as a sedative to mediate their long-term examination.
The nasal passage, pharynx, larynx and trachea up to the carina were examined to record any structural and functional abnormalities concerning the guttural pouch entry and drainage. To perform the endoscopic visualization of the guttural pouch through the working channel, biopsy forceps were used as a guide to pass through the cartilaginous flap. The endoscope was then pushed forward and rotated to roll the fibrocartilage axially, which enabled the endoscope to enter smoothly. As the endoscope passed through the opening, the salpingopharyngeal fold was clearly recognized in Figure 1a, 1b, 1c and 1d.
Figure 1: Showing the Technique Applied for Guttural Pouch Entry
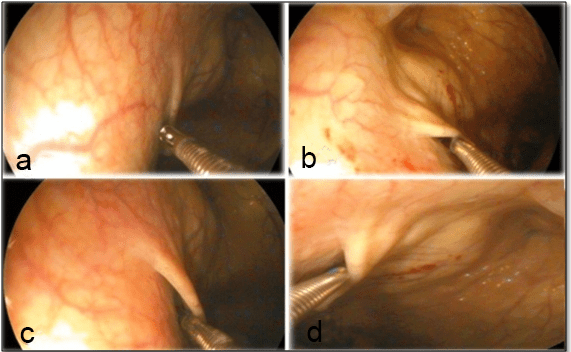
a. A Biopsy Forceps was Approached to the Cartilaginous Flap
b. The Biopsy Forceps was Inserted Underneath the Cartilaginous Flap
c. Opening of the Cartilaginous Flap to Enter the Guttural Pouch.
d. The Biopsy Forceps Introduced into the Guttural Pouch.
The endoscope brush was inserted into the orifice of Foley catheter until the tip of the catheter. The stinted Foley catheter was introduced into the nasopharynx through the nasal cavity contralateral to the side of the distended guttural pouch as demonstrated in Figure 2a. The endoscope was introduced through the ipsilateral nasal cavity until the pharyngeal ostium to visualize the affected guttural pouch. Using the endoscopic biopsy forceps, the working channel was taken out and the tip of the Foley catheter was passed through the pharyngeal ostium of the guttural pouch such that the balloon on the Foley catheter was distended with water or saline solution as in Figure 2b. The catheter was left in situ in a donkey and in a foal as demonstrated in Figure 2c and 2d.
Figure 2: Illustrating the Foley Catheter Placement for Drainage of Guttural Pouch
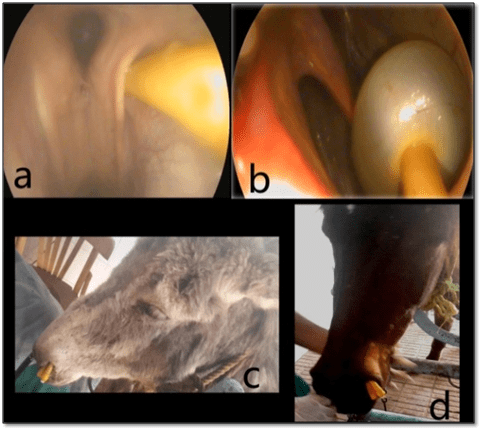
a. The Foley Catheter was Introduced into Cartilaginous Flap.
b. The Foley Catheter was Inflated
c. The Foley Catheter was Exteriorized from the Nostrils of a donkey.
d. The Foley Catheter was Placed Out of a horse Nostril.
The degree of pharyngeal lymphoid hyperplasia (PLH) was graded as: Grade 0 – a few small white follicles over the dorsal pharyngeal wall; Grade 1 – mainly small white follicles with occasional larger pink follicles over the dorsal pharyngeal wall and extending laterally to the level of the guttural pouch ostia; Grade 2 – overing pink and white follicles covering the entire dorsal and lateral pharyngeal walls and often also involving the dorsal surface of the soft palate; and Grade 3 – large pink,edematous follicles covering all visible mucosa of the pharynx and sometimes including polyps according to the criteria described by Raker & Boles.7
The degree of laryngeal hemiplasia (LH) was graded as Grade 0 – synchronous full abduction and adduction of the left and right arytenoid cartilages; Grade 1 indicates asynchronous movement (hesitation, flutter, adductor weakness) of the left arytenoid cartilage during any phase of respiration. Full abduction of the left arytenoid cartilage is (as compared to the right) inducible by nasal occlusion or swallowing. Grade 2 indicates asynchronous movement (hesitation, flutter, adductor weakness) of the left arytenoid cartilage during any phase of respiration. Full abduction of the left arytenoid cartilage cannot be induced and maintained by nasal occlusion or swallowing. Grade 3 is marked by asymmetry of the larynx at rest and no substantial movement of the left arytenoid cartilage during any phase of respiration; and Grade 4 is characterized by laryngeal asynchrony or asymmetry not included in any one of the previous grades.8
The degree of dorsal displacement of soft palate (DDSP) was graded according to the following criteria: Grade 0 – displacement is not elicited by swallowing; Grade 1 – displacement is elicited by swallowing, but is restored to the normal position after one subsequent swallowing movement; Grade 2 – displacement is elicited by swallowing, but is restored to the normal position after two or more subsequent swallowing movements; Grade 3 – displacement occurs easily in no association with swallowing. The degree of epiglottic entrapment (EE) was graded according to the following criteria: Grade 0 – not found; Grade 1 – the lateral part of the epiglottis alone is covered with the arytenoepiglottic fold; Grade 2 – the tip of the epiglottis alone is covered with the arytenoepiglottic fold; Grade 3 – almost the entire epiglottis is covered with the arytenoepiglottic fold, the assessment of appearance of disorders other than upper respiratory tract disorders was depended upon the description of.9
The endoscopic biopsy samples and the specimens of humane euthanized animals were fixed in formol saline 10%, washed, dehydrated, cleared, embedded in paraffin wax sectioned and stained with hematoxylin and eosin (H&E) following,10 then examined microscopically.
Sterile swab was taken from the growth in the palatopharyngeal fossa of a stallion and sent to the laboratory. Slide culture technique was used to detect fungal morphology after growth in Sabouraud dextrose agar plate.11
All data were collected and evaluated statistically by using an Microsoft Office Excel package 2007.12
RESULTS
Clinical Appraisal
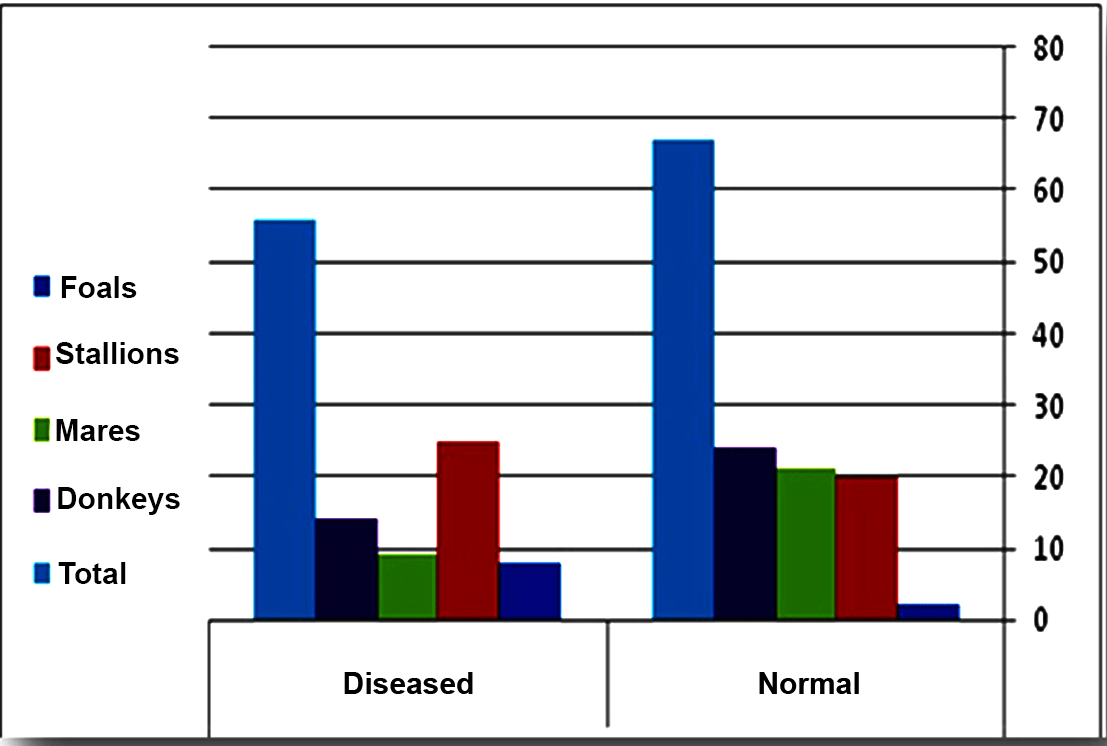
The endoscopic examination of the 123 animals reported were categorized into 56 diseased animals constituting a percentage of (45.5%), encompassing 25 stallions (44.6%), 9 mares (16%), 8 foals (14.4%) and 14 donkeys (25%). The remaining 67 animals constituting a percentage of (54.5%) were found to be endoscopically normal; including 20 stallions (29.9%), 21 mares (31.3%), 2 foals (3%), and 24 donkeys (35.8%) as shown in Graph 1. The number, age and body weight of the animals in the study were recorded in Table 1. The lesions detected during endoscopic examination were shown in Table 2. In addition, the lesions encountered in donkeys were demonstrated in Table 3.
Graph 1: Showing the Percentage of the Diseased and the Normal Animals
| Table 2: Showing the Lesions Encountered in Animals of Study. |
| No. of Stallions |
No. of Mares |
No. of Foals |
Lesions |
| 3 |
– |
– |
Epiglotic entrapment |
| 1 |
– |
– |
Dorsal displacement of soft palate |
| 6 |
2 |
– |
Pharyngitis |
| 1 |
– |
– |
Fungal growth in the palatopharyngeal recess |
| 1 |
– |
– |
Squamous cell carcinoma in guttural pouch |
| – |
1 |
– |
Cyst formation in guttural pouch |
| 1 |
– |
– |
Fibrocartilagenous mass in the trachea |
| 1 |
1 |
– |
Enlarged tracheal spike |
| 1 |
1 |
1 |
Lymphoid hyperplasia |
| 9 |
3 |
– |
Recurrent laryngeal neuropathy |
| – |
1 |
2 |
Pharyngitis and tracheitis |
| 1 |
– |
2 |
Ethmoidal Fibromatous swelling |
| – |
– |
1 |
Guttural pouch tympanitis |
| – |
– |
1 |
Rostral displacement pharyngeal arch |
| – |
– |
1 |
Ethmoidal glandular cystic acini |
| Table 3: Demonstrating the Lesions Encountered in Donkeys. |
| No. of donkeys |
Lesions |
| 1 |
Tracheal collapse |
| 13 |
Pharyngitis and tracheitis |
Disorders of Ethmoid Bone
Ethmoidal fibromatous swelling: The 2 foals in the diseased conditions constitute (14.4%) of the affected foals and 1 stallion represents (44.6%) of the total number of diseased stallions. Endoscopically, the ethmoidal fibromatous swellings were marked as lesions originating from the ethmoidal labyrinth at the caudal aspect of the middle meatus. The lesion grew rostrally into the nasopharynx. The histopathological picture of these swellings showed normal histological structure of the cartilage (a), bone (b), and the fibromatous growth (c) represented by the swelling as displayed in Figure 3.
Figure 3: Showing the Endoscopic View of the Cystic Dilatation in the Ethamoid Bone
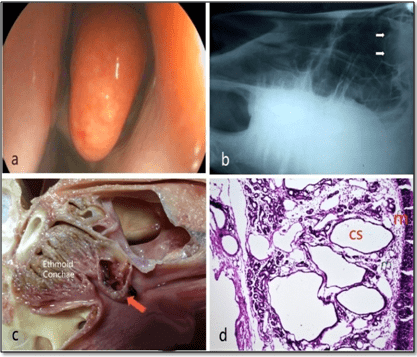
(a), the Lateral Radiographic Picture (b) (White Arrows), the PM Depiction (Red Arrow) (c) and the Cystic Dilatation (cs) of the Glandular Acini (d).
Ethmoid glandular cyst acini: In Figure (4), the right nasal passage mucosal lining was found to be swollen in the region of the middle meatus anterior to the turbinate and this swelling prevented viewing of the ethmoid turbinate via the middle meatus as a large mass protruded below the base of the middle nasal ethmoid conchae (a). Slight trauma to this structure by the end of the endoscope induced further hemorrhage. The lateral radiographic projection, revealed presence of ovoid-shaped densities super-imposed to the ethmoid region that confirmed the location and extent of this mass (b). On performing post-mortem examination, the ethmoid indicated the presence of the remnants of clotted blood and a thin lining membrane (c). On the histopathological section (d) the ethmoid cystic dilatation presented in the glandular acini (cs) with inflammatory cells infiltration (m) at the lamina propria of mucosa H&E×16.
Figure 4: Illustrating Ethmoidal Fibromatous Swelling in a Yearling foal (A) and the Histopathological Section (B)
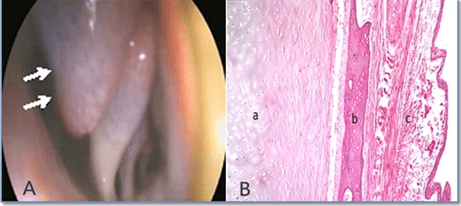
Showing the Normal Cartilage (a), Normal Bone (b), and the Fibromatous Growth (c).
Pharyngeal Disorders
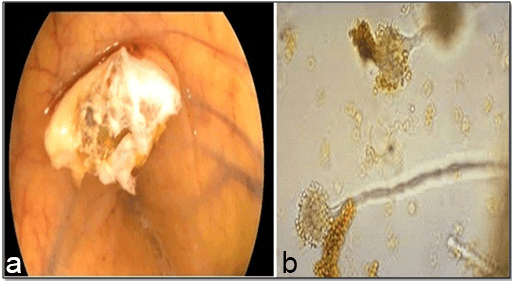
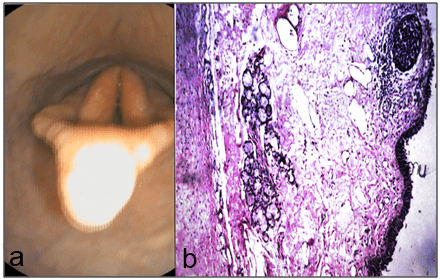
Pharyngeal mycosis (Asperiglosis): During pharyngeoscopy of a senile stallion (Figure 5), a whitish growth was observed in the palatopharyngeal recess (a). Microbiological examination of the collected samples revealed the presence of Aspergillus sp. Hyphae (b). During the PM examination, this was considered as a fistula between the guttural pouches and the dorsal pharyngeal recess as a sequel to the guttural pouch mycosis.
Figure 5: Showing the Endoscopic View of Fungal Growth (a) and Aspergillus sp. Hyphae in Culture (b).
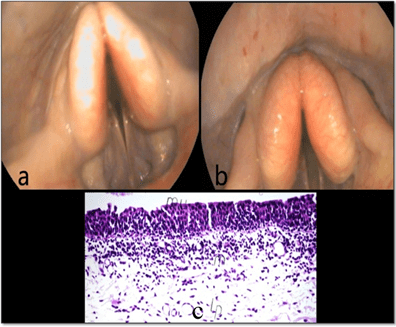
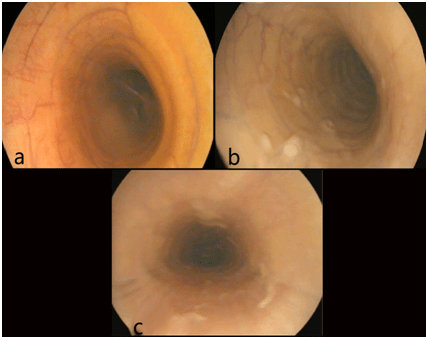
Lymphoid hyperplasia: In a horse and a foal, grade 1 lymphoid hyperplasia was characterized by the presence of small white follicles in multiple areas over the dorsal pharyngeal wall as demonstrated in Figure 6a. In a horse, grade 2 lymphoid hyperplasia was characterized by many small white lymphoid follicles located over the dorsal and lateral walls of the pharynx and extended to the level of the guttural pouch opening. They were marked by pink and glistening follicles as displayed in Figure 6b. In a horse, grade (3) lymphoid hyperplasia presented as many large coalescing follicles covering the lateral and dorsal pharyngeal wall in some of the soft palate as shown in Figure 6c. In another horse, grade (4) lymphoid hyperplasia was marked by pink, glistening follicles covering the pharynx and the dorsal surface of the nasopharynx such that these follicles coalesced into larger follicles as shown in Figure 6d. The histopathological examination of these follicles revealed abundant amount of lymphocytic cell aggregations as displayed in Figure 6e.
Figure 6: Demonstrating Grades of Lymphoid Follicles; Grade-1
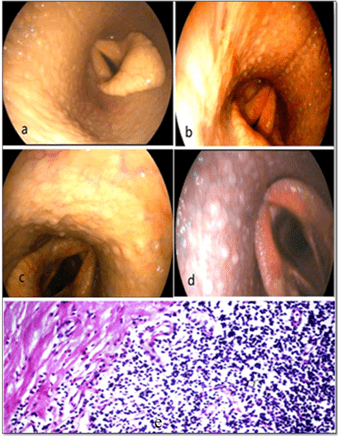
(a), Grade-2 (b), Grade-3 (c), Grade-4 (d) and the Histopathological Picture of Lymphoid Hyperplasia (e)
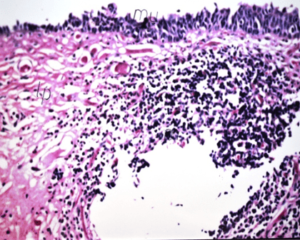
Pharyngitis: Seven horses, 14 donkeys and 1 foal suffered from pharyngitis and tracheitis indicating some mucopurulent discharge with and without hemorrhage in the nasal cavity, scattered at the conchae and or the nasal septum as illustrated in Figure 7a, 7b and 7c. The histopathological examination revealed abundant amount of inflammatory cell aggregations as shown in Figure 7d.
Figure 7: Showing the Mucopurulent Exudates in the Nasal Cavity of a Horse
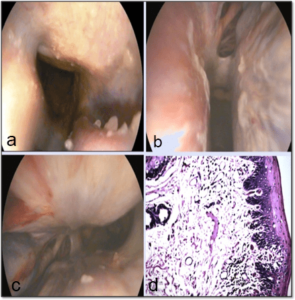
(a) a Donkey (b) and a Foal (c) and the Inflammatory Cells Infiltration in Mucosa (d) H&Ex40, Resulted from Equine Influenza.
Corniculitis: Inflammation of the corniculate processes of the larynx (Figure 8a and 8b) was observed in a donkey suffering from continuous coughing. Histopathological examination reports indicated hypertrophy in the mucosal epithelium with inflammatory cells located under the lamina propria (m) as shown in Figure 8c.
Figure 8: Showing Corniculitis (a) with Hypertrophy in Mucosal Epithelium (b) with Inflammatory Cells in Underlying Lamina Propria (mu) H&Ex40.
Epiglotitis: In a donkey, inflammation of the epiglottis was observed during examinationas is evident in Figure 9a. The mucosal layer showed normal histological structure while,indicating the presence of focal circumscribed round granuloma – like formation of lymphoid cells as displayed in Figure 9b.
Figure 9: Showing the Endoscopic Picture of Epiglottitis (a), and the Focal Lymphoid Cells
and Granuloma Formation (b) in the Lamina Propria of Mucosal Layer (m)
Pharyngeal tonsillitis: The inflammation of the lymphoid follicles was located dorsolaterally to the pharynx, between the pharyngeal openings of the auditory tubes. It appeared as a large swelling, protruded into the pharyngeal cavity as shown in figure 10a and 10b. The histopathology sections showed lymphoid hyperplasia as shown in Figure 10c.
Figure 10: Demonstrating the Various Endoscopic Views of Pharyngeal Tonsillitis (a & b) and
Histopathological Section, the Tonsil showing Lymphoid Hyperplasia (c).
Recurrent Laryngeal neuropathy (RLN): Following endoscopic examination, the grading system of arytenoid motility determines the incidence of clinical RLN. Grade 1 as in Figure 11 is viewed in 3 stallions and 1 mare where the adductory and abductory movement is asymmetric. As the endoscope is introduced through the right nasal passage, the right arytenoid may appear less abducted and vice versa. Grade 2 is endoscopically recognized in 2 stallions and 1 mare; transient flutter or delayed abduction is seen in the left arytenoid. Grade 3 is endoscopically recorded in 1 stallion and 1 mare, the left arytenoid abduction activity is reduced when compared with the right, with periods of prolonged asymmetry, particularly during quiet movements. Grade 4 is endoscopically determined in 3 stallions. The left arytenoids are not full abducted and during adduction, the right arytenoid crossing the midline with some residual movement is observed.The histopathological examination demonstrated in Figure 12 revealed normal histopathological structure of mucosa (mu) and a glandular structure (g) in grade 1 observed in the 3 stallions and a mare. The arytenoid showing focal inflammatory cells aggregation in lamina propria of mucosa in grade 2 was observed in 2 stallions and a mare (Figure 13).
Figure 11: Illustrating the Degrees of Recurrent Laryngeal Hemiplasia; First Degree (a), Second Degree (b), Third Degree (c), and Forth Degree (d).
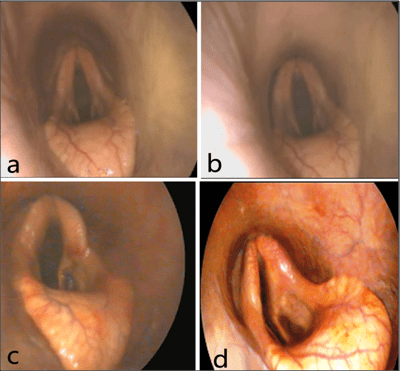
Figure 12: The Histopathological Changes of Arytenoids in Grade 1 Recurrent Laryngeal Neuropathy.
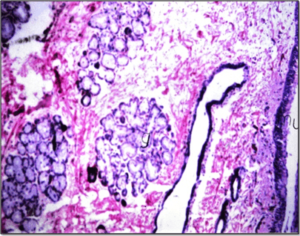
Figure 13: The Histopathological Changes of Arytenoids in Grade 2 Recurrent Laryngeal Neuropathy.
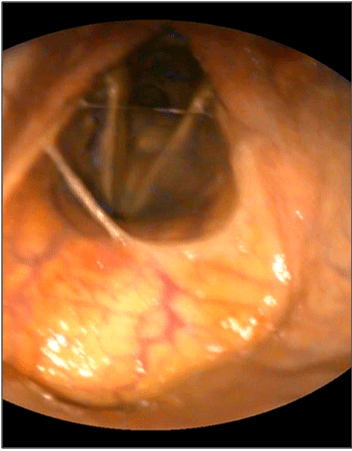
Epiglottic entrapment: In a senile horse, epiglottic entrapment was recognized during the advent of the flexible endoscope, whereas the glosso-epiglottic and aryepiglottic folds are unidentified as illustrated in Figure 14.
Figure 14: Demonstrating the Epiglottic Entrapment in a Horse
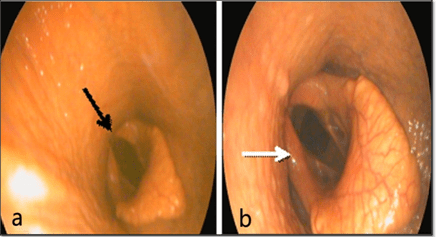
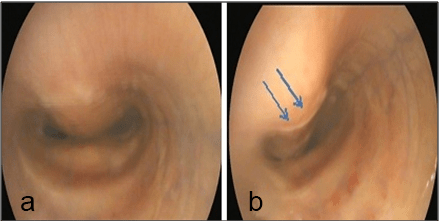
Rostral Displacement of the Palatopharyngeal Arch (RDPA): A 3-year-old foal exhibited coughing during dancing as observed by endoscopic visualization of the respiratory tract. Pharyngitis and dorsal displacement of the palatopharyngeal arch were obvious observations as shown in Figure 15.
Figure 15: Showing Rostral Displacement of the Palatopharyngeal Arch (Black Arrow) and
Adducted Position of the Right Arytenoid Cartilage (White Arrow).
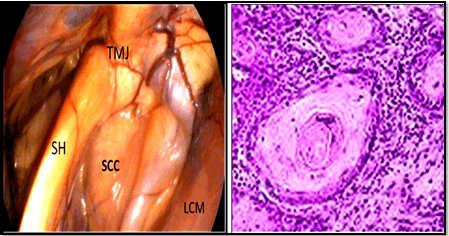
Guttural pouch squamous cell carcinoma: An old horse exhibited the clinical signs including the swelling in the parotid region, dyspnea, dysphagia, and pneumonia. The endoscopic examination of their guttural pouches revealed that one horse suffered from squamous cell carcinoma accompanied with metastasis to the retropharyngeal lymph nodes which resulted inthe enlargement and collapse of the dorsal roof of the nasopharynx and in turn led to dyspnea and dysphagia.
In Figure 16, the squamous cell carcinoma was located in between the stylohyoid bone and internal carotid artery (a). The histopathological changes included the visualization of multiple clusters of neoplastic epithelial cells with eosinophilic cytoplasm and prominent nucleoli together with abundant interstitial infiltration of lymphocytes and mono-nuclear cells (b).
Figure 16: Showing the Location of The Squamous Cell Carcinoma (a) and the Histopathological changes (b) which Revealed Multiple
Cluster of Neoplastic Epithelial Cells with Eosinophillic Cytoplasm and Prominent Nucleoli together with
Abundant Interstitial Infiltration of Lymphocytes and Mononuclear Cells.
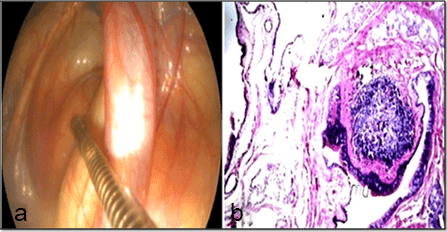
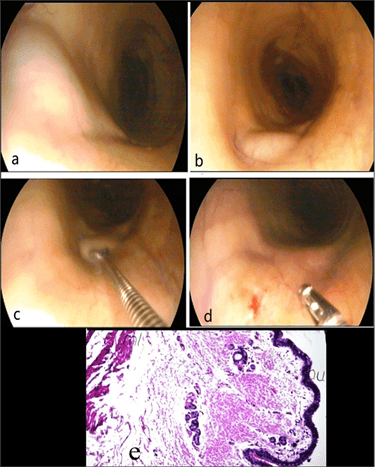
Guttural pouch cyst: Another observation showed cyst formation in the guttural pouch in Figure 17. Endoscopically, the cyst was located in the lateral compartment of the guttural pouch. It was an ovoid shaped structure that contained clear fluid inside, similar to the polyp-like mass which measured 6 cm in length and 4 cm in width and its wall was traversed by vasculatures as shown in (a). Histopathologically, the cyst was a benign growth lined with stratified squamous epithelium between 2 to 8 cells thick. Parallel to the epithelium was a dense layer of fibrous connective tissue. The fibrous connective tissue also formed loose whorls around the blood vessels and was more pronounced in the deeper tissue layer as demonstrated in (b).
Figure 17: Showing the Cystic Structure in the Lateral Compartment of the Guttural Pouch
of a Stallion (a) and the Cyst was Histopathologically lined by a Layer of Pseudostratified Epithelium (b).
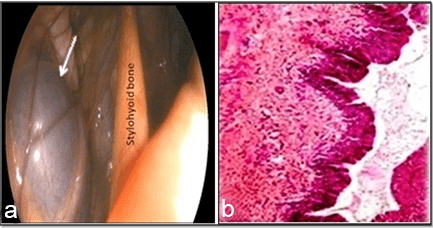
Guttural pouch granuloma: As demonstrated in Figure 18, the guttural pouch of a donkey showed a focal circumscribed round mass (a). The histopathology of the trans-endoscopic biopsy samples (b) revealed the formation of granuloma with a necrosed center (m) in the mucosal layer (mu).
Figure 18: Illustrating theGranulomatous Mass (a) and its Formation in the Histopathological Section (b) Stained with H&Ex16
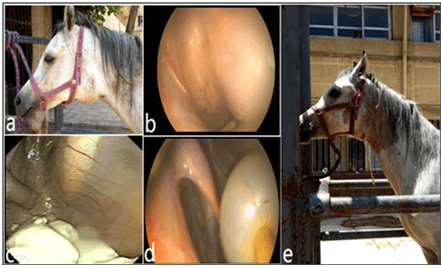
Guttural pouch tympanitis: In Figure 19, the clinical picture of a foal suffering from bilateral tympanitis was described in (a), collapse of the dorsal pharyngeal wall and compression of the nasopharynx as the lateral walls moved medially by the distention of the pouches during endoscopic examination was displayed in (b), the infected material within the left guttural pouch was illustrated in (c), and the indwelled and inflated Foley catheter was shown in (d) introduced within the pouch to facilitate irrigation and drainage via percutaneous gutturocentesis (e).
Figure 19: Showing Clinical Picture of Guttural Tympanitis (a), Endoscopy of Swelled Pouch in the Pharyngeal Cavity (b), Presence of
Pusy Material Inside the Medial Compartment (c), Applied and Inflated Foley Catheter within the Pouch (d),
and the 3-year Foal after Irrigation of the Pouch via the Indwelled Foley Catheter (e).
The tympany resolves immediately after the Foley catheter enters the guttural pouch, provided that only one guttural pouch is affected. If tympany of the contralateral guttural pouch becomes apparent after the guttural pouch was decompressed, a second catheter needed to be inserted through the pharyngeal ostium of that guttural pouch.
Disorders of Trachea
Pharyngitis and tracheitis: 1 mare, 3 foals and 10 donkeys were diagnosed with pharyngitis and tracheitis using the endoscopic examination. The affected animals showed some mucopurulent discharge with and without hemorrhage in the nasal cavity, scattered at the conchae and or the nasal septum as illustrated in Figure 20a, 20b and 20c.
Figure 20: Showing Various Forms of Tracheitis in a Mare (a), in a Foal (b), and in a Donkey (c).
Tracheal collapse: During the endoscopic examination of a donkey; the trachea appeared to be misshaped dorsoventrally and flaccid resulting in a collapse at the distal cervical part of the trachea. The circumferential or lateral luminal narrowed with stenosis as shown in Figure 21a and 21b.
Figure 21: Showing Tracheal Colapse in a Donkey (a) , and the Dorsoventral (Blue Arrows) Lumen Reduction at the Distal Cervical Part (b).
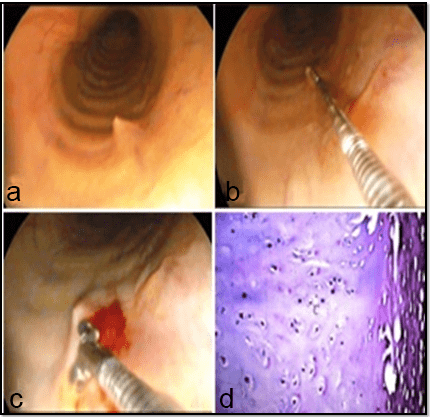
Tracheal fibroma: As shown in Figure 22, an ovoid mass (22a) was viewed by performing tracheoscopy of a stallion, as an elevated swelling in the midcervical portion of the trachea (22b),of the dimensions 1.5 cm×2 cm (width×length). The histopathological sections of the biopsy samples (22c and 22d) revealed an excessive formation of fibrocytes in the dermal layer and accumulation of inflammatory cells in the lamina propria with the intact mucosal surface (22e).
Figure 22: Showing the Ovoid Mass (a), Located at Midcervical Portion of the Trachea (b), Biopsy Sampling (c),
the Sampled Site (d), and the Fibrous Connective Tissue Formation in the Underlying Dermis,
and the Inflammatory Cells Infiltration in the Lamina Propria (e).
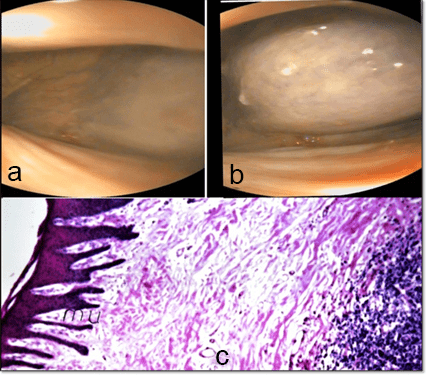
Tracheal spike: As demonstrated in Figure 23, spike-shaped granulomatous lesions appeared as sporadic nodules (23a) and were observed in the trachea of a stallion and a mare at the proximal tracheal lumen nears the larynx (23b). The histopathological examination of the collected endoscopic samples (23c) showed normal cartilaginous structure and mucous surface (23d).
Figure 23: Demonstrating the Tracheal Spike. Normal Endoscopic Appearance (a), Location of the Spike (b),
Biopsy Sampling (c), and the Normal Cartilaginous Structure (d).
DISCUSSION
The present study provided evidences of some disorders or disease conditions in 25 stallions (44.6%), 9 mares (16 %), 8 foals (14.4%), and 14 donkeys (25%) exhibited remarkable distress in the upper respiratory tract (URT). Diagnosis was based on case history, clinical appraisal, and physical examination, besides endoscopic, microbiologic and histopathologic investigations.
Donkeys are still used as work animals and play an important role in the economy of the developing countries, but surprisingly little information is available relating to the structure and functional anatomy of the upper airways of this species.13 Conversely, enormous information have been published for horses in this respect.14 Therefore the present study included both horses and donkeys to settle down the endoscopic and anatomic depictions variation.
The three cases of ethmoidal fibromatous swellings were considered an incidental visualization throughout the endoscopic examination of the upper respiratory tract in one stallion (represents 44.6% of the total diseased stallions) and two foals (constitute 14.4% of the affected foals). Endoscopically, the lesions grow rostrally into the nasopharynx, and accompanied clinically with respiratory distress and cough. However, another three cases of ethmoid swelling were diagnosed osteoma and an osseous fibroma by Cilliers et al.15
The ethmoid glandular cyst acini in a senile stallion was detected during endoscopic examination, that appeared as large mass protruded below the base of the middle nasal ethmoid conchae. Radiologically and histopathologically revealed ethmoid cystic dilatations with glandular acini with inflammatory cells infiltration in between the lamina propria of mucosa. Similar findings have been reported in a senile stallion by endoscopic visualization and radiographic examination.16,17 Furthermore, this lesion is not likely a neoplastic process as it does not demonstrate uncontrolled, progressive cell multiplication, which is a hallmark of neoplasia as mentioned by Bell et al.18
Pharyngeal asperiglosis in the palatopharyngeal recess has been observed during pharyngeoscopy in a stallion based on the microbiological examination. Similar lesion was located more anteriorly and resulted in the development of a fistula between the right and left guttural pouches and the dorsal pharyngeal recess as reported by Jacobs and Fretz.19
Pharyngeal lymphoid hyperplasia (PLH) is a condition occurs in horses involving proliferation of one of the equine tonsillar structures. In the present work, it has been diagnosed in a stallion, two foals, and a mare during endoscopic examination.20 It was obvious that the pharyngeal tonsil was first effects when upper respiratory infections occurred. This might be due to its common access to pathogens either through inhalation via the nasopharynx or ingested through the oropharynx. During the acute phase, the follicles were edematous and hyperemic, with slight ulceration on the most prominent portion. The accompanying nasal discharge is initially serous, then converted into mucopurulent. Concurrently, pharyngeal follicular hyperplasia involves the proliferation primarily of one of the five tonsillar structures in the equine. Its anatomical placement and diffuse nature predisposes the pharyngeal tonsil to inflammation. This inflammation begins acutely due to viral agents and progresses to a chronic nature by the addition of air turbulence and mechanical irritation, secondary bacterial invaders, and caustic agents such as smoke inhalation. Clinically, the most common signs are inspiratory dyspnea and exercise intolerance. Endoscopic exam reveals varying grades of hyperplasia, from a few scattered, white follicles to hyperemic, edematous follicles and polyps involving the entire visible pharyngeal mucosa.21
Pharyngitis has been recognized in five senile stallions and two old mares. Although it is very common in young horses, where its prevalence decreases as horses become old and develop immunity.22 Pharyngitis is an airway inflammation. It is more common in horses and caused by infection that originated due to bacteria and viruses, as well as environmental agents such as endotoxin, ammonia, respirable dust, and metal.2 It is important to evaluate the extent of pharyngeal inflammation, which classified into four grades based on the degree of severity.23,24 Endoscopic examination of diseases of the upper airway associated with poor racing performance.25
The pharyngeal tonsils are lymphoid-filled follicles on the dorsum of the pharynx are most often seen in young horses, their number and size decreasing at 2 to 3 years of age, and only a few being present at 4 or 5 years.26
The etiology of epiglottitis is unknown. But possible predisposing factors include pharyngeal inflammation, intermittent dorsal displacement of the soft palate, epiglottic entrapment, and trauma from ingestion of foreign bodies.27
Endoscopically, epiglottitis results in edema, reddening, and thickening of the epiglottis and aryepiglottic fold. Extensive swelling and marked discoloration of the mucosal tissue that attaches loosely to the lingual surface of the epiglottis is common, and swelling of the lingual surface may cause mild to marked dorsal elevation of the epiglottic axis. Cartilage at the tip of the epiglottis may be exposed, resulting in granulation tissue formation. Chondritis of the epiglottic cartilage can develop and may result in epiglottic deformity during healing.28
Tympany is usually unilateral, but bilateral cases have been reported, diagnosis is based on clinical examination, endoscopy, with or without radiography, or percutaneous gutturocentesis. The latter technique should be performed with great care, as there is risk of damage to nervous and vascular tissues.29
Recurrent laryngeal neuropathy (RLN) is considered one of the upper airway obstructive diseases. The knowledge of its etiology, pathogenesis, methods of assessment and the critical evaluation of treatment.30 True hemiplegia, where one-half of the larynx, almost invariably the left side, shows no active abductory or adductory motility, can be determined easily at rest. However, controversy still exists regarding the clinical significance of asymmetric or asynchronous movements of the arytenoid cartilages, it is more common in large breed-horses and has been associated with the height of horses.31 However, the disease is considered to affect predominantly large young male animals.32 A horse with small epiglottis can perform well and a horse with a very robust large looking epiglottis can still displace its palate.33
The principle means of differentiating entrapment of the epiglottis by a redundant arytenoepiglottic fold from elongation of the soft palate is through endoscopic examination of the larynx and pharynx. When entrapment of the epiglottis occurs the arytenoepiglottic folds become redundant and curl over the epiglottis obscuring only the anterior border of the epiglottis from the view of the observer. However, when elongation of the soft palate occurs the epiglottis is lying ventral to the soft palate and it is therefore completely lost from the observer’s view.34
Rostral displacement of the palatopharyngeal arch (RDPA), over one or both of the corniculate processes of the arytenoids that appears malformed, does not abduct well, or both. Abnormal arytenoid movement in most cases might be a result of abnormal anatomy of laryngeal cartilages and muscles, not restriction by the palatopharyngeal arch. The long-term prognosis for horses with this problem is poor, these coincide with.35
Horses with DDSP described as making a characteristic gurgling sound during expiration.1 Recently, it has suggested that up to 30% of horses with DDSP make no audible abnormal respiratory sounds at exercise.36
The tracheal spikes have recognized by endoscopic viewing and proved normal by the histopathological examination. Moreover, the etiology of these spikes is unknown and not associated with clinical signs.37
The endoscopic findings showed tracheal collapse in a donkey as dorso-ventral flattening of tracheal ring in the thoracic part of the trachea and this result is agreed with findings of Ohnesorge et al.38 Also, it was found in thorax of ponies and donkeys that resulted from dorsal ligament flaccidity.39 It occurred after traumatic damage to cartilage rings or the intrathoracic portion of the trachea showed transient collapse in horses affected with severe pulmonary disease as result of raised intrathoracic pressures.40 However, chronic airway disease, malformation of the hyaline cartilage rings, or trauma resulted in abnormal collapse of the tracheal lumen.41 Tracheal collapse in older donkeys because of age related degeneration in tracheal ring/cartilage or secondary to other respiratory diseases that caused an increase in respiratory effort; typically collapse occurred in animals with chronic recurrent airway obstruction or pulmonary fibrosis.42
CONCLUSION
The present investigation recommends the use of video-endoscopy for regular examination, assessment and tracing the health for appropriate diagnosis and management of upper respiratory system in horses and donkeys. The horse’s upper respiratory disorders are numerous and more popular than donkey’s disorders. Moreover, the present study has proved the occurrence and the superiority of pharyngeal disorders in horses than donkeys. Endoscopic examination of guttural pouches needs the use of biopsy instrument as a guide to allow advance of the endoscope through the auditory tube and into guttural pouch. The presence of squamous cell carcinoma and cyst formation in the guttural pouches of horses and granuloma in the guttural pouch of a donkey has claimed to be foremost records.
Future studies on endoscopic evaluation of respiratory system and application of accessories are hoping to ameliorate the surgical interferences of such upper respiratory tract disorders in horses and donkeys.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests and no financial interests related to the substance of this manuscript.





























