DEFINITION AND EPIDEMIOLOGY OF
ENDOMETRIOSIS
Endometriosis is a condition where endometrial glands and endometrial stroma are present outside the uterus. It is common with prevalence between 10 and 15% in females of reproductive age, especially between the ages of 30 and 40.1 However, it can also affect prepubertal, pubertal and postmenopausal women. Endometriosis is classified as a benign inflammatory process that may be asymptomatic or may cause a variety of symptoms, ranging from chronic vague pelvic pain to dysmenorrhea, menorrhagia, dyspareunia, dyschezia, and dysuria, etc.
The location of the disease is often seen in the pelvis, and implantations are seen along the peritoneum, the outer surface of the uterus, the fallopian tubes and the ovaries. However, extra-pelvic locations exist but are less frequent. Endometriosis implantations have been reported on the bowels, the diaphragm, subcutaneous tissue, pleural cavity, brain, lungs, lymphatic system, etc.
Hormonal fluctuations lead to the growth of lesions, shedding and bleeding during the menstrual cycle. Then end result is thought to be inflammation and irritation of the surrounding tissues leading to adhesions.
PATHOGENESIS OF ENDOMETRIOSIS
Several hypotheses have been proposed to explain the pathogenesis of endometriosis (Table 1).
| Table 1. Pathogenesis of Endometriosis |
| Retrograde menstruation (Sampson’s theory) |
Viable endometrial tissue fragments spread from the uterine cavity to the peritoneal cavity |
| Endometrial stem cell implantation |
Endometrial epithelial progenitor cells regurgitated into the peritoneum |
| Metaplastic transformation (Meyer’s theory) |
Coelomic epithelium undergoing a metaplastic transformation to endometrial-like tissue |
| Hormones |
Increased responsiveness to estrogen, progesterone resistance |
| Mullerian remnant abnormalities |
Abnormal differentiation and/or migration of the cells of the Mullerian ducts |
| Oxidative stress and inflammation |
Lipoproteins peroxidation, DNA damage, hemoglobin breakdown, local inflammation |
| Immune dysfunction |
Defective immune surveillance, modified HLA expression |
| Apoptosis suppression, alteration of cell fate |
Anti-apoptotic pro-proliferative phenotype, angiogenesis |
| Genetics |
Genes promoting attachment to the peritoneum and evasion from immune cells |
Retrograde Menstruation (Sampson’s Theory)
According to Sampson, viable endometrial tissue fragments are spread from the uterine cavity to the fallopian tubes and then to the peritoneal cavity, by a gradient of pressure due to the dyssynergic contractions of the uterus. In the peritoneal cavity, these fragments may implant and grow with the hormonal changes.
This sequence is increased with regular abundant menstrual flows, in the setting of decreased age at menarche, number of pregnancies, breastfeeding duration. However, many women were found with retrograde menstruation, but without implantation of endometrial cells in the peritoneal cavity. This raises the possibility of other factors to explain the implantation of cells other than just a pure mechanical retrograde menstruation.1
Endometrial Stem Cell Implantation
This theory is an extension of the retrograde menstruation theory, suggesting that endometrial epithelial progenitor cells, as well as mesenchymal stem-cell-like cells, are regurgitated through the fallopian tubes into the peritoneum, accompanied by their niche cells. These cells lead then to ectopic endometrial implants.1
Metaplastic Transformation (Meyer’s Theory)
This concept suggests that the coelomic epithelium, which is the embryologic precursor of both peritoneal and endometrial tissues, undergo a metaplastic transformation from peritoneal cells to endometrial-like tissue. Hormonal, biochemical and/or immunological factors may be the origin of this metaplasia.2
Hormones
Several studies suggested that ectopic endometrial tissues have an increased responsiveness to estrogen and that several environmental toxins (dioxin for example) may interact with estrogen receptors, mimicking its activity.
These ectopic tissues express reduced 17β-hydroxysteroid enzymes, leading to a less conversion of estradiol to the less potent estrone. They have, as well, a certain resistance to progesterone causing a prolonged proliferative activity as compared to eutopic tissues.3
Mullerian Remnant Abnormalities
This theory suggests that abnormal differentiation and/or migration of the cells of the Mullerian ducts may cause the spread of these cells in the pathway of organogenesis during fetal life, over the posterior part of the pelvic floor.
This is mostly valid for endometriotic lesions seen in the uterosacral ligaments and in the cul-de-sac.1
Oxidative Stress and Inflammation
Lipoproteins peroxidation by reactive oxygen species leads to DNA damage in the endometrial cells. These oxygen species may originate from the water and electrolytes found in the peritoneal fluid.
Hemoglobin breakdown in the peritoneal cavity increases iron levels, which causes redox reactions as well.
Local inflammation leads to lymphocytes recruitment and macrophages activation, increasing the secretion of cytokines that cause oxidation of enzymes and promotion of endometrial cells growth.3
Immune Dysfunction
The recurrent endometrial cells regurgitation into the peritoneal cavity and the recurrent activation of inflammatory cells leads to a defective immune surveillance and a defective elimination of endometrial cells that will implant and grow.4
Endometrial cells in the peritoneal cavity may, in addition, modify the expression of HLA molecules, acquiring resistance to immune-mediated lyses.5
Apoptosis Suppression and Alteration of Endometrial Cell Fate
Several in vitro studies proved a modification of the fate of the endometrial cells to an anti-apoptotic and pro-proliferative phenotype. These changes favor the survival of these cells in the ectopic sites.
This modification is facilitated by the activation of genes that stimulate cell proliferation, inflammation, and angiogenesis.6
Genetics
The genetics theory is supported by the familial aggregation of endometriosis, especially between twins. Several chromosomal loci were stated to be associated with endometriosis, using different techniques (gene arrays, laser capture microdissection, comparative genomic hybridization, etc).
Genes involved helped the development of endometriosis by promoting the attachment between these cells and the peritoneum as well as their evasion from immune cells.7
Having all these theories in mind, no one can explain all aspects of the disease. Also, the disparity between the stage of endometriosis according to the revised American Fertility Society (r-AFS) classification and the severity of pain or infertility raises the question about potential other explanation for this disease.
One of the recent advances in biomolecular medicine is epigenetics. Though there is no clear definition of epigenetics, it can be considered as the study of stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence. Epigenetic modifications can be passed from one cell generation to the next (mitotic inheritance) and between generations of a species (meiotic inheritance).
In the following part of the article, the possibility of epigenetics influence in the field of endometriosis will be explored as to how it affects the pathogenesis of the disease.
EPIGENETICS OF ENDOMETRIOSIS
Alteration of DNA Methylation
DNA methylation regulates chromatin remodelling as well as transcription networks. Several factors have been shown to affect DNA methylation such as age, inflammation, diet, hypoxia, etc.
Hypomethylation and hypermethylation of DNA lead to phenotypic changes on several levels, mainly hormonal, biochemical, immunological, apoptotic and angiogenic. These changes will cause estrogen excess, progesterone resistance, apoptosis alteration (Table 2).
| Table 2. DNA Hypo/Hyper Methylation |
| Hypomethylation |
Hypermethylation |
| CYP19 (aromatase cytochrome P450) |
17β-hydroxysteroid dehydrogenase type II |
| Steroidogenic acute regulatory protein (StAR) |
Progesterone receptor – B (PR-B) |
| Estrogen receptor – β (ERβ) |
E-cadherin |
| Steroidogenic factor – 1 (SF-1) |
Homeobox A10 (HOXA10) |
| Cyclo-oxygenase-2 (COX-2) |
GATA2 |
| GATA6 |
|
Methylation of the 5-carbon position of the pyrimidine ring of cytosines, paired with a complimentary guanosine (CpG sites) creates CpG islands in gene promoters. This modification leads to transcription suppression causing silencing of the corresponding gene. This process of methylation is stable, yet reversible.8
5-methylcytosine is catalyzed by a group of DNA methyltransferases (DNMT). DNMT 1 maintains DNA methylation, whereas DNMT 3A and 3B cause de novo DNA methylation.9
Both hypomethylation and hypermethylation of specific genes have been implicated in endometriosis, with a dominance of hypomethylation due to the downregulation of DNMT1.
Hypoxia causes an increase in AU-rich element binding factor 1 (AUF1) and microRNA-148a (miR-148a) leading to lower levels of DNMT1 (Protein and mRNA) in ectopic endometrial cells.10
Inflammation is another microenvironmental factor implicated in the development of endometriosis.
Increased prostaglandin E2 (PGE2) in chronic inflammation stimulates DNMT3a, leading to hypermethylation of specific loci.
These changes are modulated by several factors (integrins, matrix metalloproteinases, tissue inhibitors of metalloproteinases, apoptosis pathways, etc).11
The pathway of steroid hormones is also affected by epigenetic dysregulation in endometriosis.
In fact, DNA coding for proteins such as CYP19 (aromatase cytochrome P450) and steroidogenic acute regulatory protein (StAR) and for nuclear receptors such as estrogen receptor-β (ER-β) and steroidogenic factor-1 (SF-1) are hypomethylated in endometriosis. This process leads to an increased 17β-estradiol biosynthesis.12
In parallel, the promoter of cyclooxygenase-2 (COX-2) is hypomethylated, leading to an increased production of COX-2, which increases PGE2 causing higher levels of 17β-estradiol and DNMT3a in ectopic endometrial tissue.13
DNA hypermethylation enhances the bioavailability of 17β-estradiol, by inhibiting the enzyme that converts this form of estrogen to the less potent form (17β-hydroxysteroid dehydrogenase type II).14
Chronic inflammation causes prolonged stimulation by tumor necrosis factor alpha (TNF-α) which is a proinflammatory cytokine leading to hypermethylation of the promoter region of progesterone receptor-B (PR-B) in endometrial cells and therefore downregulation of this receptor and progesterone resistance.15,16
E-cadherin is a transmembrane glycoprotein that forms adherence junctions between cells. Hypermethylation of the E-cadherin gene in the promoter region results in decreased expression in endometriotic cells, and subsequently, increased invasiveness of these cells.17
Homeobox A10 (HOXA10) is a transcription factor important for endometrial development. Hypermethylation of the promoter region of HOXA10 gene (progesterone-responsive gene) in the eutopic endometrial cells of patients with endometriosis causes downregulation of this factor, increasing progesterone resistance.18
GATA factors are transcription factors that bind to the “GATA” DNA sequence and control cellular maturation, proliferation and survival by activating or repressing transcription. Genes that code for GATA factors were also found to be differentially methylated in women with endometriosis.
GATA2, which regulates genes needed for the differentiation of healthy endometrial cells, is hypermethylated in endometriotic cells, causing a downregulation of its function.
Whereas, GATA6, which regulates genes allowing the expression of aromatase and other enzymes necessary to increase estrogen availability and progesterone resistance, is hypomethylated in endometriosis, causing an upregulation of its function.19
Dysregulation of micro RNA (miRNA) Expression
Micro RNAs are single-stranded non-coding RNAs that regulate gene expression by pairing with mRNAs to modulate RNA translation, degradation, and splicing. These regulations have a significant impact on cell growth, apoptosis, signalling, etc.
Endometrial cells were found to have downregulation of miRNAs that target several factors20:
– mediators of inflammation: cyclooxygenase-2, interleukin-6, and interleukin-6 receptors
– inducer of apoptosis: B-cell lymphoma-2
– cycle regulator: cyclin D1
– angiogenic factors: vascular endothelial growth factor, interleukin-8
Hypermethylation of DNA in endometrial cells causes suppression of expression of miR-23a and miR-23b, and consequently, increased expression of SF-1, StAR, and CYP19, granting these cells increased steroidogenic capacity.21
Hypoxia induces overexpression of miR-20a which downregulates dual specificity phosphatase 2 (DUSP2), leading to an extended extracellular signal-regulated kinase (ERK) phosphorylation. These changes lead to an increased PGE2 bioavailability.22
MicroRNA-148a is elevated in ectopic endometrial cells, suppressing the expression of DNMT1 and leading to a global hypomethylation.10
Inflammatory cytokines induce upregulation of miR-302a, suppressing chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) causing increased PGE2 and therefore increased DNMT3a and DNA hypermethylation.10
Recent studies reported also hypermethylation of miR-196b and miR-503, causing downregulation of these microRNAs, and subsequent increase in expression of genes involved in cellular development, anti-apoptosis and angiogenesis (c-myc, cyclin D1, Bcl-2, VEGF, etc).23
Immunologic Dysregulation
The difference between the uterine immune status of women with and without endometriosis is involved in the pathogenesis of this disease (Table 3).
|
Table 3. Immunologic Dysregulation
|
|
↑ Fas ligand on endometriotic cells and in peritoneal fluid → ↓ T-lymphocytes toxicity
|
|
↑ FoxP3 positive T regulatory cells →↓ ability of immune cells to target shed cells
|
|
↓ Natural killer cells cytotoxicity
|
|
↓ Fas-mediated apoptosis
|
|
↑ Growth factors, macrophages, B-lymphocytes and pro-inflammatory cytokines
|
|
↑ Autoantibody production
|
|
↑ B-cell lymphoma/leukemia – 2 (Bcl-2) and ↓ Bcl-2 associated X protein (BAX)
|
|
↑ Nuclear factor kappa B (NFϰB) → ↑ cellular growth, ↓ apoptosis
|
The main immunologic dysregulations that occur during endometriosis are increased growth factors and pro-inflammatory cytokines, increased availability of macrophages and B-lymphocytes, and decreased availability of natural killer cells.24
Abnormal miRNA expression and subsequent DNA hypomethylation cause T-cell activation leading to a breakdown of T-cell and B-cell tolerance and autoantibody production.25
FoxP3 gene, which controls the function of T regulatory (Treg) cells, is more expressed on Treg cells in the endometrium of women with endometriosis.
These FoxP3+ Treg cells downregulate the ability of some immune cells to target shed viable endometrial cells.26
Fas ligand (FasL) which is expressed on endometriotic cells binds to Fas expressed on lymphocytes leading to the apoptosis of T-lymphocytes.
Furthermore, the level of FasL is increased in the peritoneal fluid of patients with endometriosis.
These 2 changes lead to a reduction in the cytotoxicity of T-lymphocytes and an increase in the survival of shed endometrial cells.27
Another immunologic dysregulation in endometriosis is an increased expression of B-cell lymphoma/leukemia-2 (Bcl-2) which is an anti-apoptotic factor and a decreased expression of Bcl-2 associated X protein (BAX) which is a pro-apoptotic factor.28
Nuclear factor kappa B (NFκB) expression is increased in endometriosis, stimulating cell growth, and inhibiting apoptosis.29
Alteration in Histone Methylation
Histone aberrant methylation has also been shown to be involved in the pathogenesis of endometriosis.
Estradiol (E2) and progesterone (P4) activate histone deacetylase 1 and 2 (HDAC 1 and 2) leading to histone hypoacetylation (lower levels of H3K9ac and H4K16ac) which further causes downregulation of estrogen receptor-α (ER-α).30
Increased PGE2 upregulates histone acetyltransferases (SRC-1, p300, and CBP) causing endometriotic cells to be hypermethylated at H3K4, H3K9, and H3K27. These changes cause increased expression of SF-1 and StAR in endometriosis.31
Another study showed that H3K27me3-positive nuclei (trimethylated histone 3 at lysine residue 27) are more prevalent in endometriotic cells compared to eutopic endometrial cells. Moreover, endometriotic cells have higher levels of EZH2 (enhancer of zester homolog 2) than eutopic endometrial cells. This protein is an enzyme that causes trimethylation of H3K27me3 and is upregulated by progesterone.32
CONCLUSION
Endometriosis is a common condition especially in females of reproductive age. Several hypotheses have been proposed to explain the pathogenesis of endometriosis: retrograde menstruation (Sampson’s theory), endometrial stem cell implantation, metaplastic transformation (Meyer’s theory), hormonal changes, mullerian remnant abnormalities, oxidative stress, inflammation, immune dysfunction, apoptosis suppression, alteration of endometrial cell fate and genetic changes.
The disparity between the stage of this disease and the severity of its symptoms raises the question about potential other explanation for this disease.
Researchers made many studies regarding the possibility of epigenetic influence in the field of endometriosis.
DNA methylation, micro RNA expression, immunologic system, and histone methylation are the main systems involved in epigenetic alterations in endometriosis (Figure 1).
Figure 1. Epigenetics of endometriosis
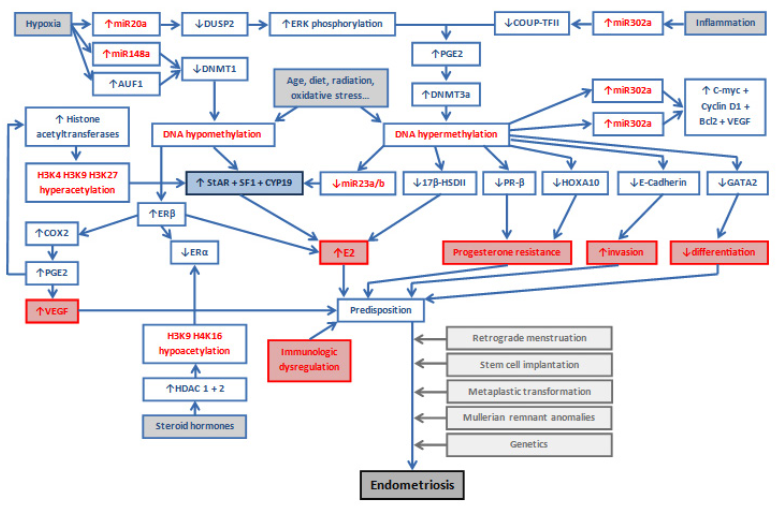
DNA: deoxyribonucleic acid; RNA: ribonucleic acid; 17β-HSDII: 17β-hydroxysteroid dehydrogenase II; DNMT: DNA methyltransferase; miR: microRNA; AUF1: AU-rich element binding factor 1; PGE2: prostaglandin E2; CYP19: aromatase cytochrome P450; StAR: steroidogenic acute regulatory protein; ERβ: estrogen receptor – β; SF-1: steroidogenic factor – 1; COX-2: cyclooxygenase – 2; PR-B: progesterone receptor – B; HOXA10: Homeobox A10; DUSP2: dual specificity phosphatase 2; ERK: extracellular signal-regulated kinase; COUP-TFII: chicken ovalbumin
upstream promoter – transcription factor II; Treg: T regulatory; Bcl-2: B cell lymphoma/leukemia – 2; HDAC: histone deacetylase; ERβ: estrogen receptor – β.
These alterations might be the target of new more effective treatments in this field.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.






