INTRODUCTION
The acute hepatopancreatic necrosis disease (AHPND) has affected shrimp farming in several countries, like, Vietnam, Malaysia, Thailand, Mexico and in Philipines.1,2,3,4,5,6 The unique symptoms and characteristics of this disease consisting of massive sloughing of hepatopancreas epithelial cells.4 The external symptoms in infected shrimp like, empty stomach, bluish body color and shrunken hepatopancreas could be observed. The rate of mortality is significantly higher on the 1st 3 days of infection. The AHPND appear in the culture ponds from 8-45 days of stocking.
The Vibrio parahaemolyticus was identified as causative agent of AHPND by Tran et al.7 It carries a plasmid (pAP1) of approximately 69 kbp. This plasmid contains 2 genes which produce toxins. These 2 genes act together to cause AHPND in shrimp. The susceptible known species are Penaeus vannamei, P. monodon and P. chinensis.8
There are several efforts done to minimize the effect of pathogen using herbal products. Other authors9 showed the antimicrobial effects of 2 essential oils (EOs) Vibrio concentrations in the rearing water of Penaeus monodon.
The proposed trial was designed with the objective to determine the efficacy of essential oil enriched shrimp feed against Vibrio parahemolyticus in Penaeus vannamei.
MATERIALS AND METHODS
Bioassay Lab and Glass Aquaria
A bioassay trial was set-up using 6 aquaria each for experimental groups as well as for control groups. The specific pathogen free (SPF) juvenile shrimp 10 each in number were maintained in 6 aquaria each. Each aquarium was filled with 5 litre of seawater provided with required dissolved oxygen (DO) supply.
Location and Time Period
The experiment was conducted in bio-secured laboratories at Ben Tre Aquaculture Station, Binh Dai, and Extension Department, Vietnam in the period of September 16, 2014 to October 30, 2014.
Experimental Shrimp
Shrimp of mean body weight 0.23 to 0.33 g were used to conduct the trial. The available size of SPF Shrimp (PL 10) were procured from a bio-secured Hatchery (Ca Dec Seed Production Centre, Ben Tre, Vietnam) and reared in 500-litre capacity tank for 10-15 days.
Shrimp Screening and Acclimatization
The shrimp were screened at Government disease diagnostic center (RAHO), Ho Chi Minh City and CÔNG TY CO PHAN DICH VU THUY SAN THANH LOAN Disease Diagnosis Lab at Ho Chi Minh City for pathogen of shrimp i.e. white spot syndrome virus (WSSV), Infectious myonecrosis virus (IMNV), infectious hypodermal and haematopoietic necrosis virus (IHHNV), taura syndrome virus (TSV), yellow head virus (YHV) and Vibrio spp. prior to start of the trial.
Shrimp Food
Shrimp feed were produced at Feedmill, Lampung of PT. Central Proteinaprima Tbk. The feed types were as followed, post-larvae feed (PL 03:250-400 micron) and shrimp feed (CP 001:0.425-0.75 mm and CP 02:0.71-1 mm) as per the requirement of experimental shrimp. The anti-AHPND essential oil formulation were developed by combining the essential oil blend extracted from the following 10 plants, Lavandula latifolia, Pinus sylvestris, Jasminum officinale, Citrus limon, Prunus avium, Viola odorata, Gardenia jasminoides, Cocos nucifera, Rosa damascene and Eucalyptus globulus. Using expeller-pressing method (Anderson International Corp, OH, USA) performed the oil extractions from the selected plants.10 The essential oil blend were mixed with the feed in required amount. The basic formulation of both the feed was same except essential oil mixed in the experimental feed.
AHPND Disease Challenge Procedure
The immersion method of challenge was applied in the trial.11 The isolated purified bacteria, Vibrio parahemolyticus, strain VP A/3 (procured in August 2014 from University of Arizona, USA) were utilized for the trial. The V. parahemolyticus was grown up to the density of 107 CFU/ml in Tryptose Soy Broth (TSB) and then 5 ml of it was poured in each tank. The final V. parahemolyticus concentration in the tank water was 105 CFU/ml. The 5 ml blank TSB was applied in the negative control tanks (Table 1).
| Table 1: Experimental design of immersion challenge trial. |
|
Group
|
Replicate |
MBW (gr) |
Density/5 L |
Bacteria Density
(CFU/mL) |
Challenge methods |
Feed |
Water Exchange |
| Positive Control |
6 |
0.23-0.33 |
5 |
107 |
Pour 5 mL of Vibrio
parahaemolyticus in each tank |
Regular |
20%/ day (no water exchange during challenge)
|
|
Treatment
|
Essential oil enriched
|
|
Negative Control
|
Pour 5 mL of sterile TSB
solution in each tank
|
Regular
|
Post-Challenge Observation
EMS gross sign observation: The challenged shrimp were observed for body color, hepatopancreas color and shape and feed consumption rate and cumulative mortality. The severity level of infection was categorized into:
- Medium level G2: Hepatopancreas color light pale, yellow within hepatopancreas connective tissue capsule, size of hepatopancreas normal and no sign of atrophy;
- Severe level G3: Hepatopancreas color pale, yellow or white within hepatopancreas connective tissue capsule, significant sign of atrophy in hepatopancreas (shrunken).
Lab confirmation: The AHPND was confirmed by typical gross sign appearance, by polymerase chain reaction (PCR) analysis and sequencing analysis.
Vibrio parahaemolyticus Isolation and Sequencing Analysis
The Vibrio parahaemolyticus was isolated from the hepatopancreas of challenged shrimp using Chrom-Vibrio agar. The colonies of V. parahaemolyticus spp. appeared mauve color. Further, sequencing of obtained V. parahaemolyticus was carried out. The primers utilized were AP3 Reverse and AP3 Forward12 with a base length of 236 bp. The analysis of sequencing was performed at First Base, Singapore.
Statistical analysis: Statistical analysis were done by analysis of variance (ANOVA) with p<0.05 confidence level.
RESULTS
Shrimp Gross Sign Appearance
The positive control shrimp started showing the clear symptoms of AHPND after 18 to 22 hour of challenge. The stomach was empty and significant drop in the lipid droplets in hepatopancreas.
Feeding Rate
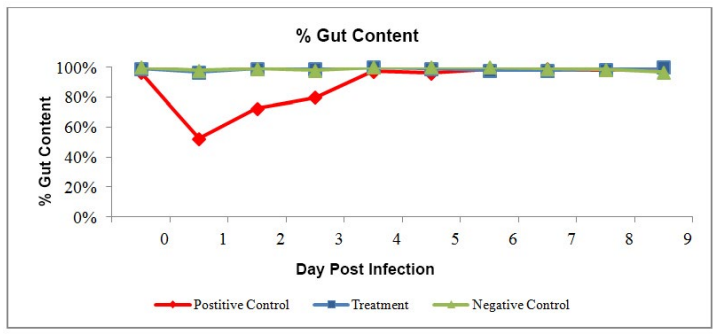
The feed consumption dropped significantly after challenge in positive control (about 50%). It showed the stress in shrimp. The shrimp start recovering in the treatment group from dpi 3 onwards (Figure 1).
Figure 1: Daily feed consumption rate in percentage (%).
Cumulative Mortality
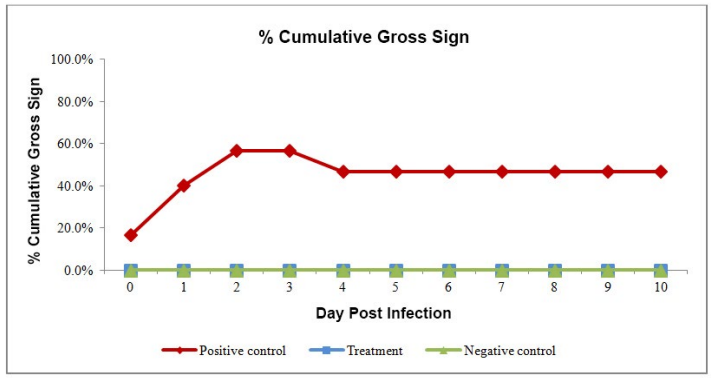
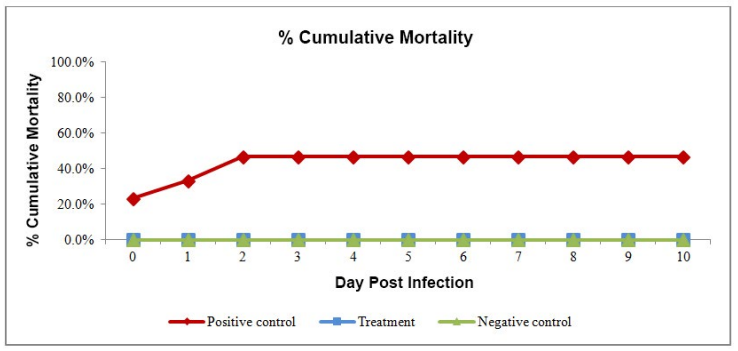
The cumulative gross sign appearance in positive control reached up to 56% by day 3 of challenge (Figure 2). The typical gross sign of AHPND, like, shrinkage of hepatopancreas, reduction in lipid content and empty stomach, started appearing from the day of challenge. The cumulative mortality rate was 46.7% at dpi 10 after challenge in positive control whereas no mortality in treatment group and in the negative control group (Figure 3). The moribund and dead shrimp had clear symptoms of AHPND.
Figure 2: Cumulative gross sign percentage of AHPND in trial groups.
Vibrio parahaemolyticus Isolation and Basic Local Alignment Search Tool (BLAST) and Sequence Alignment
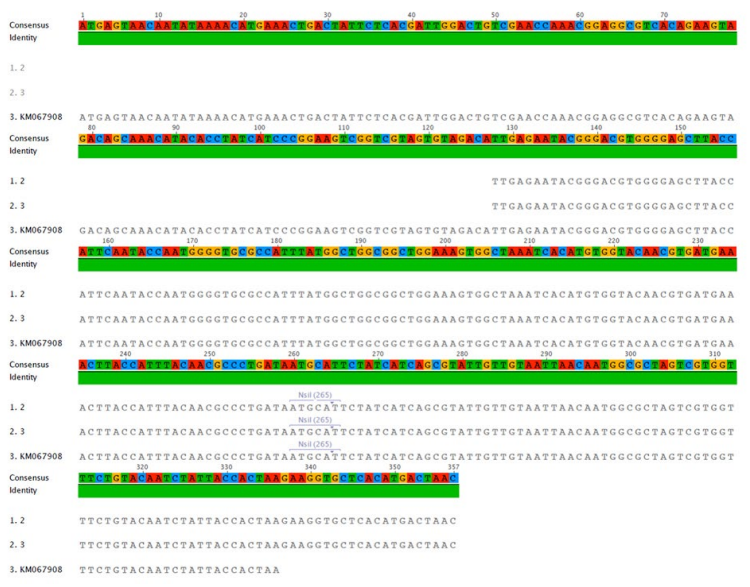
The extracted Vibrio parahaemolyticus from the stomach of positive control shrimp were sequenced (Table 2) and aligned (Figure 4).
Figure 4: Result of BLAST and alignment analysis.
| Table 2: Sequences producing significant alignment National Center for Biotechnology Information (NCBI) blast. |
|
Description
|
Max Score |
Total Score |
Query Cover |
E value |
Ident |
Accesion
|
| Vibrio parahaemolyticus strain 3 HP plasmid pVA1, complete sequence |
425
|
425 |
99% |
2e-115 |
100% |
KP324996.1
|
| Vibrio parahaemolyticus genes for hypothetical proteins, JHE-like toxin PirA-like, JHE-like toxin PirB-like, complete cds |
425
|
425 |
99% |
2e-115 |
100% |
AB972427.1
|
| Vibrio parahaemolyticus strain 13-028/A3 plasmid pVPA3-1, complete sequence |
425
|
425 |
99% |
2e-115 |
100% |
KM067908.1
|
| Vibrio parahaemolyticus strain 20130629002S01 putative VP19 protein (vp19) gene, complete cds |
425
|
425 |
99% |
2e-115 |
100% |
KM035408.1
|
| Vibrio parahaemolyticus plasmid pVPA3-1 DNA, putative toxin region |
348
|
348 |
84% |
3e-92 |
99% |
LC032040.1
|
The results of BLAST and alignment analysis from National Center for Biotechnology Information (NCBI) showed that the sample was identical to accession number KM067908.1 (Figure 3) that is Vibrio parahaemolyticus 13-028/A3 and 13-028/A2. Strain 13-028/A3 was determined to cause this disease through laboratory bioassays (Table 3).7
Figure 3: Cumulative mortality percentage of trial groups.
| Table 3: Statistical analysis of AHPND challanged treatment and control groups using ANOVA |
|
Mortality
|
| Duncana |
|
|
Subset for alpha=.05 |
|
Treatment
|
N |
1 |
2 |
| Treatment Feed Group
Negative Control Group
Positive Control Group
Sig. |
6
6
6 |
.0000
.0000
1.000 |
46.6667
1.000
|
| Means for groups in homogeneous subset are displayed. aUses Harmoni Mean Sample Size = 6.000. |
Statistical Analysis
There was significant difference between treatment and positive control in terms of cumulative mortality.
DISCUSSION
The AHPND has appeared in almost all major shrimp producing countries of South East Asia. Much effort has been made to minimize the effect of disease by application of various available products like, probiotics, bacteriophages, immunostimulants, herbal extracts, quorum quenching, acidifiers and toxin absorbents, etc., with potential of anti-AHPND properties. However, most of them could not achieve the successful outcome as per expectation. The combinations of 10 natural oils were formulated to develop as anti-AHPND in the present study. The selection of oil for the formulation was done on the basis of their anti-viral and anti-bacterial properties. The product is combination of blend essential oils. The artificial feed as a carrier of anti-AHPND product is one of the best ways to provide the protection to the shrimp. Vietnam is one of the major suffering countries due to AHPND and so was selected for the trial site.
The final laboratory results provide conclusive evidence that in a controlled environment anti-AHPND feed provide prevention against AHPND-Vibrio parahemolyticus. The treatment shrimp that ingested AHPND infected tissue did not develop AHPND and did not show gross signs and no mortality during the experimental period. However, in contrast the shrimps in the positive control group demonstrated up to 46.7% mortality with clear symptoms of AHPND. The AHPND gross-sign appearance in the challenged shrimp was similar and as described by NACA, OIE, Tran et al.13,14,7 There was feed drop on first 3 days of challenge in positive control, which indicates that the digestive system of the challenged animals without protection was damaged. The immersion method of challenge using purified and certified pathogenic Vibrio parahemolyticus procured from University of Arizona was selected to avoid any risk of contamination. The rate of mortality in the challenged group was similar to the field reports i.e. heavy mortality for 3 to 5 days with significant drop in feed intake. The bioassay challenge trial conducted by Tran et al7 received the similar rate of mortality as recorded in the positive control of the current trial. The Vibrio parahemolyticus has short generation time which facilitates colonization and result of which large number of cells can dominate the host in a short period of time, giving it advantage to dominate over other bacteria in the surrounding environment.8,15
The sequencing alignment analysis results showed that the isolated V. parahemolyticus causing typical symptoms of AHPND is 100% matching with the strain isolated by Tran et al and Lightner DV.7,16 The core genome of all V. parahemolyticus strains is composed of 3,284 genes, 24.3% of total pangenome. The core genome of only the pathogenic strains has 3,358 genes (FAO 2016).8 Whereas, there are possibilities of more than one strain of V. parahemolyticus or isolates of other Vibrio species carrying the pathogenic genes (PIR A and PIR B) to cause symptoms like AHPND in shrimp.8,17,18
CONCLUSION
The obtained results and the analysis shows that the shrimp fed on blended oil mixed with feed have significant protection against AHPND-Vibrio parahemolyticus. The next step would be to conduct field trials in culture ponds to determine the efficacy of developed feed against AHPND.
AUTHORʼS ACKNOWLEDGEMENTS
We would like to thank PT. Central Proteina Prima, Indonesia and Panacea Natural Sciences, Indonesia to provide funding for the research. We thank Directorate of Fisheries, Government of Vietnam, providing facilities, guidance and special assistance in conducting the bioassay trial. We also would like to thank Prof. Don Lightner, University of Arizona, USA for providing the AHPND strain of Vibrio parahemolyticus.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









