INTRODUCTION
Androgenetic alopecia (AGA) is a partially genetically predetermined and progressive form of hair loss that affects approximately 50% of men and women.1 In men this occurs largely after puberty, the majority of women affected by female-pattern baldness have reached menopause.
While chronic inflammatory stress may facilitate androgenic alopecia,2,3 AGA is largely driven by the androgen dihydrotestosterone (DHT). Both DHT, and the enzyme responsible for converting testosterone to DHT, 5α-reductase, are increased in the bald scalp.4 Also elevated in bald scalp are prostaglandin D2 synthase (PTGDS) and its enzymatic product, prostaglandin D2 (PGD2), indicating a relationship between hair loss and these components.5
Hair loss can cause significant psychological distress to those affected and many seek treatment to address it.6 The most commonly available topical treatments for AGA, Finasteride and Minoxidil, are also available in oral formulations which offer convenience but are generally less effective.7 Finasteride is a type 2 5α-reductase inhibitor which reduces the conversion of testosterone to DHT, thereby lowering levels of DHT in the scalp.8 While the mechanism of action of Minoxidil is still largely unclear, it is thought enhance hair cycling by shortening the telogen or resting phase of hair growth and prolonging the anagen or active growth phase.9 Finasteride and Minoxidil have shown success as hair loss treatments, but both are associated with adverse effects which constrain their long-term oral use.
Other topical treatments such as cetirizine10 and saw palmetto11 show promise, but there is inadequate evidence concerning their oral efficacy and there are safety concerns i.e., with cetirizine. More invasive treatments such as micro-needling, various lasers, platelet-rich plasma and adipose-derived stem cells are generally used in clinic settings.
Ageratum conyzoides (A. conyzoides) Linn, also known as billy goat weed, is a tropical plant originating in Africa. Traditionally, this herb has been used to treat skin disorders, headache, rheumatism and wound healing.12,13 We previously demonstrated the efficacy of a topical formulation of A. conyzoides in the treatment of hair loss. Our open label study provided evidence for facilitating hair growth and reducing hair loss in men and women following 8 weeks of daily topical application.14 This was corroborated by a 12-week randomised, double-blind clinical study which confirmed that the daily application of a topical A. conyzoides formulation resulted in a net increase in hair growth.15
The pragmatic use of A. conyzoides as a hair loss treatment is supported by a robust safety profile. The topical formulation described above, a pyrrolizidine-free extract of the herb, showed no evidence of mutagenicity, clastogenicity or in vivo genotoxicity.16 Fetotoxicity and teratological toxicity investigations provided further reassurance.17
Given the efficacy of a topical formulation of A. conyzoides in improving hair growth and reducing hair loss, this follow-up study sought to investigate the efficacy and safety of an oral formulation.
MATERIALS AND METHODS
This clinical study was a randomised, double-blind trial investigating the efficacy of an orally administered A. conyzoides formulation on increasing hair growth and decreasing hair loss in males and females over a 12-week period. Participants in the study included males and females over the age of 18, reporting hair loss. Reasons for exclusion from this study were: clinically significant medical conditions including, but not limited to, cardiovascular, neurological, psychiatric, renal, immunological, endocrine (including uncontrolled diabetes or thyroid disease) or haematological abnormalities that are uncontrolled; scalp conditions or any other genetic disease or issue that could contribute to baldness; current use or use of hair growth formulations in the past three months; previous use of radiotherapy to scalp for cancer treatment; current or past history of Cicatricial alopecia; females with clinical diagnosis of menstrual and/or endocrine disorders, polycystic ovary syndrome (PCOS) or hyper-androgenism; previous or continued use of antihypertensives, steroids, spironolactone, ketoconazole, cytotoxic compounds, anticonvulsant drugs, testosterone or testosterone boosting supplements, oestrogens or progesterone within the last six months; participation in another hair growth trial 3-months before the start date of this study; significant recent alterations in hair style; females not on a suitable form of birth control, or pregnant, up to 12-months postpartum or lactating; specific hair styles (dreadlocks, afro) which made quantitative monitoring difficult, and anyone who had undergone considerable colouring, bleaching, straightening or curling.
Study participants were randomised to receive the investigational product A. conyzoides 250 mg capsule, or an oral placebo (250 mg capsule containing microcystalline cellulose and maltodextrin). Participants were required to consume the allocated product once a day with 250 mL of water, at breakfast. Prior to treatment, participants attended the clinic to undertake assessments including baseline measures of hair densitometry (using the HairCheck® device), hair comb/count test, hair tug/pull test, and a blood test.
The primary outcome of this study was change in hair growth over 12-weeks. This included investigating hair densitometry by the HairCheck® instrument, and a hairline recession assessment. Other measures included the Norwood/Hamilton and Ludwig-Savin scales, assessing male-pattern baldness and female pattern baldness, respectively.
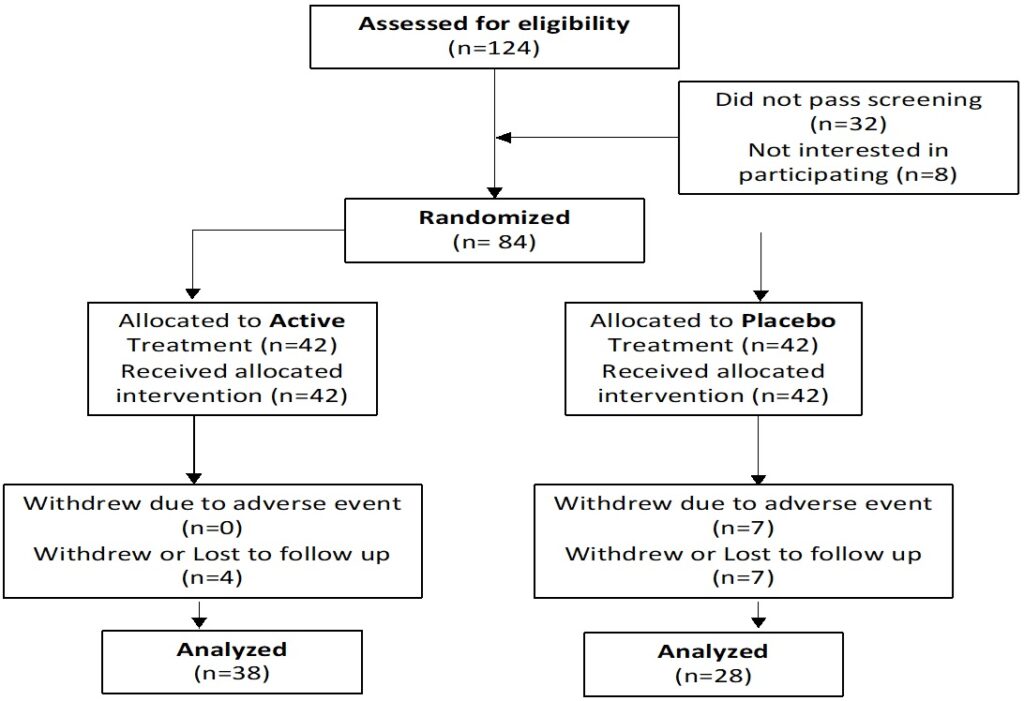
Hair loss was assessed by the mean number of hairs lost in a one-minute combing test (>150 hairs lost was deemed “poor”; 100-150 hairs lost, “fair” and 50-100 hairs, “good”), and a hair tug/ pull test. Blood tests were taken at baseline and after 12-weeks of treatment to determine changes in serum markers including DHT, insulin-like growth factor 1 (IGF-1), high-density lipoprotein (HDL), Erdr1, PTGDS, PGDT, GPR44, full blood count (FBC), E/LFT, thyroid stimulating hormone (TSH), ferritin and Vitamin D. Quality-of-Life assessments were reported via a self-evaluated questionnaire (Figure 1).
Figure 1. Participant Flow Chart
Statistical Analysis
Statistical analysis was conducted through analysis of variance (ANOVA) and t-tests or Mann-Whitney tests to compare change from baseline over 12-weeks treatment as well as comparison between groups. Statistical significance was defined as p<0.05.
RESULTS
A total of 84 participants were enrolled in this study. Eighteen (18) dropped out; 7 due to adverse events in both groups, and 11 were lost to follow-up.
Baseline Demographics
There were no statistically significant differences between groups at baseline or week 4, 8 or 12 for any of the demographic or anthropometric measures above (Table 1).
| Table 1. Participant Details |
|
A. conyzoides (n=38)
|
Placebo (n=28)
|
| Age (years) |
44.0 (10.7)
|
42.8 (12.8)
|
| Gender |
16 females, 22 males
|
5 females, 23 males
|
| Systolic BP |
122.5 (15.3)
|
124.7 (11.8)
|
| Diastolic BP |
80.7 (10.8)
|
79.7 (9.6)
|
| Heart rate |
69.7 (11.7)
|
71.0 (11.6)
|
| Weight (kg) |
81.0 (17.2)
|
84.9 (23.3)
|
| Height (cm) |
1.73 (0.10)
|
1.78 (0.08)
|
| BMI kg/m2 |
27.2 (5.8)
|
26.4 (5.6)
|
| Data presented as mean (standard deviation) |
Hair Growth
Hair density: The HairCheck® device measures hair density using cross-section trichometry.18 The density of hair in a thinning area of the scalp is compared to that of a non-thinning area to generate the hair loss ratio. After 12-weeks of treatment, there was a trend toward a significant increase in hair density in the A. conyzoides group compared to the placebo group (p=0.08). There was an increase in hair density in the A. conyzoides group compared to baseline. The placebo group, on the other hand, showed a decrease in hair density compared to baseline. There was no statistically significant difference in hair density ratio between treatment groups at any time point (Table 2).
| Table 2. Hair Density |
|
A. conyzoides
|
Placebo
|
|
Baseline
|
Week 12
|
Baseline
|
Week 12
|
| Control |
79
|
|
80
|
|
| Hair thickness |
38.23
|
39.62
|
29.85
|
28.59
|
| Change in thickness |
|
1.38
|
|
-1.26
|
Hairline assessment: The hairline assessment measures the distance from the eyebrow to the start of the hairline. After 12-weeks of treatment, there was a statistically significant difference between groups, with a lower distance in the A. conyzoides group (Table 3).
| Table 3. Hairline Assessment |
|
Baseline
|
Week 4
|
Week 8
|
Week 12
|
A. conyzoides
Total Hairline measure |
7.90 (2.1)
|
7.91 (2.2)
|
7.85 (2.1)
|
7.82 (2.1)*
(p=0.02)
|
Placebo
Total Hairline measure |
8.94 (2.1)
|
8.88 (2.4)
|
8.91 (2.1)
|
9.06 (1.8)
|
| Data presented as mean (standard deviation) |
Other hair measures: Evaluation of the Norwood/Hamilton (for men) and Ludwig Savin (for women) scales, and hair loss measures showed no significant differences in either group from baseline or between groups.
Quality-of-life: No statistically significant differences were found between groups for the quality-of-life (QoL) questionnaire.
Biochemical and Haematological Analysis
Biochemical and haematological parameters were measured at baseline and week 12 to detect change from baseline value. There were no significant differences between groups at baseline in any of the biochemical or haematological markers. There was a statistically significant difference in change from baseline for alkaline phosphatase (ALP) and prostaglandins at week 12 between active and placebo groups. In males, a statistically significant change was found in 5α-reductase-2 expression after 12-weeks of treatment (Table 4).
| Table 4. Biochemical and Haematological Analysis |
|
Baseline
|
Change from baseline
|
|
A. conyzoides
|
Placebo
|
A. conyzoides
|
Placebo
|
| 25 OH Vit D (ng/mL) |
28.1
|
26.1
|
1.2
|
0.4
|
| IGF-1 (ng/mL) |
170.4
|
181.4
|
-4.3
|
-11.4
|
| Testosterone (ng/mL) |
3.2
|
4.3
|
0.0
|
0.4
|
| TSH (mIU/L) |
2.0
|
2.2
|
0.1
|
-0.1
|
| Albumin (g/L) |
44.7
|
45.5
|
-0.1
|
0.1
|
| ALP (U/L) |
90.1
|
77.9
|
-13.3*
(p=0.013)
|
7.8
|
| ALT (U/L) |
21.3
|
23.3
|
2.0
|
-1.8
|
| AST (U/L) |
26.8
|
29.4
|
1.1
|
0.1
|
| Cholesterol (mmol/L) |
5.9
|
5.3
|
0.1
|
0.2
|
| Ferritin (ng/mL) |
118.5
|
146.4
|
13.3
|
8.3
|
| HDL (mmol/L) |
1.6
|
1.5
|
0.0
|
0.0
|
| LDL (mmol/L) |
3.9
|
3.3
|
0.1
|
0.1
|
| Triglycerides (mmol/L) |
1.3
|
1.4
|
-0.1
|
-0.3
|
| Total Protein (g/L) |
71.7
|
73.2
|
-0.5
|
-0.6
|
| GGT (U/L) |
25.3
|
24.7
|
0.7
|
5.1
|
| Bilirubin (umol/L) |
9.2
|
7.8
|
-1.1
|
-3.0
|
| Creatinine (umol/L) |
69.3
|
78.2
|
7.0
|
7.4
|
| Glucose (mmol/L) |
6.2
|
6.1
|
-0.4
|
-0.2
|
| Total Prostaglandins (pg/mL) |
14.6
|
22.5
|
-1.5*
(p=0.016)
|
7.4
|
| DHT (pg/mL) |
257.5
|
298.1
|
6.2
|
22.6
|
| 5a-reductase-2 (pg/mL) |
963.6
|
1288.0
|
-33.2
|
97.1
|
| Males only: |
| 5a-reductase-2 (pg/mL) |
1456.6
|
1421.5
|
-107.6*
(p=0.043)
|
94.0
|
| *significant difference between groups |
Adverse Events
Eight adverse events were reported. Two were reported in the active group (abdominal pain, gas) and three in the placebo group (loose stools, abdominal pain and nausea). With the limited number of adverse events and similar occurrence between groups, the gastrointestinal (GI) symptoms do not appear to be related to the product. The remaining three adverse events (urinary tract infection, other infection and muscle spasm) were experienced by the placebo group.
DISCUSSION
The results of this clinical study add to the body of work demonstrating the efficacy of A. conyzoides on improving hair growth and reducing hair loss. The 12-week daily supplementation of an oral formulation of A. conyzoides improved growth at the hairline and increased hair density as measured by the HairCheck® device. Whilst hair density measures did not reach statistical significance, a trend toward significance was observed.
Critically, the investigational product in this study was administered orally. Most treatments for hair loss involve topical application. These generally show greater efficacy than oral formulations7 for primarily pharmacokinetic reasons, i.e., by delivering higher concentrations of the active(s) directly to affected areas of the scalp. An A. conyzoides formulation administered topically had indeed been more rapidly effective, with significantly improved hair growth by 8-weeks of treatment.15 Accordingly, we believe that extended use of oral A. conyzoides may yield further improvements in hair density.
In measures of hair loss and assessments through the Norwood/Hamilton and Ludwig-Savin scales, there were no statistically significant differences between groups. No significant differences were found between groups in assessment of the QoL questionnaires.
We have previously shown in in vitro investigations that A. conyzoides extract inhibits the expression of type 1 5α-reductase and PGD2 release in human hair dermal papilla cells.14 A similar reduction in type-1 5α-reductase following A. conyzoides treatment has been shown in human prostate cells.19
In the present study there was a significant difference in change from baseline in total prostaglandins between groups at week 12. In the active group total prostaglandins were reduced at week 12 from baseline, while they increased in the placebo group. Similarly, in males at week 12, type 2 5α-reductase expression was reduced in the active group whilst increasing in the placebo group. Here too, the change from baseline between groups was statistically significant. While these results are in line with previous evidence of the mechanism of action of A. conyzoides in targeting hair growth, the documented anti-inflammatory properties of A. conyzoides20 may have contributed to the overall effect. There was also a significant difference in change from baseline in Alkaline phosphatase between groups at week 12, with a decrease in the active group and increase in the placebo group. Assessment of other biochemical and haematological measures showed no significant differences between groups.
The results of this study, together with our previous clinical and in vitro studies demonstrate the safety and efficacy of A. conyzoides in improving hair growth, with observed reductions in expression of type 2 5α-reductase and prostaglandins pointing to a clear mechanism of action.
CONCLUSION
Due to the importance of healthy hair in terms of body image and self-esteem, hair loss can cause intense distress. There is consequent demand for safe and effective treatments to address hair loss. We have previously shown that a topical A. conyzoides formulation improved hair growth and was safe when supplemented daily for 12-weeks. This follow-up investigation demonstrated the efficacy of an oral formulation, complementing and extending the results from our previous studies. Further trials will determine whether the effects on hair growth are sustained over the longer-term.
We cannot at this stage say what happened after the 12-weeks, as Ethics Committee approval was restricted to a 12- week trial. Further trials will-determine whether the effects on hair growth are sustained over the longer-term, before decisions can be made regarding commercialization.
COMPLIANCE
This trial was conducted in compliance with the current International Conference on Harmonization (ICH) Guideline for Good Clinical Practice (GCP), the Therapeutic Goods Administration (TGA) Note for Guidance on Good Clinical Practice, and ethical guidelines outlined in Additional Ethical Considerations. The ethics committee which approved the trial is Bellberry Ltd, Approval number 2018-04-235-PRE-3.
AUTHORS’ CONTRIBUTIONS
Amands Rao supervised and coordinated the clinical trial. Paul Clayton and Nathasha Bogoda wrote and edited the paper.
CONFLICTS OF INTEREST
Nathasha Bogoda is an employee of Gencor Pacific, Paul Clayton provides occasional consultancy services. RDC Global is an independent clinical research organisation.






