INTRODUCTION
Comparative studies of estimated multiple myeloma morbidity rate by age group have reported that morbidity increases with age.1 The main treatment modalities offered for elderly patients include the conventional melphalan prednisolone (MP) strategy and new drugs such as bortezomib (Bor) and lenalidomide (Len). Melphalan-prednisone-thalidomide (MPT), which consists of MP with addition of thalidomide (T) has improved the prognosis in elderly patients.
However, although newly introduced drugs have improved treatment outcomes, the elderly often have concomitant diseases or lowered physical functions and quality of life (QoL), and require special help, such as transportation to medical facilities. Steroids are another important drug in treating multiple myeloma, and can increase efficiency on multiple myeloma when combined with new drugs. On the other hand, long-term use of steroids in elderly patients often causes adverse events and complications. The E4A03 trial comparing lenalidomide plus high dose dexamethasone (LD) and lenalidomide plus low-dose dexamethasone (Ld) therapy also recommends the low-dose administration of steroids.2 They advocate low-dose guidelines, particularly since adverse events of steroids often make long-term treatment with steroids difficult in the elderly.3,4 Thus, the lenalidomide plus prednisolone (RP) therapy which replaces low-titer steroid prednisolone (PRD) with dexamethasone is a potential new treatment option enabling safe and long-term treatment for elderly patients.
Foreign clinical trials have reported the efficacy of lenalidomide and RP therapy in treating multiple myeloma patients,5 whereas no data on efficacy and safety on Japanese patients are yet available. Therefore, we retrospectively assessed the efficacy and safety of RP therapy in elderly patients with multiple myeloma and report herein.
SUBJECT AND METHODS
We retrospectively analyzed 22 elderly relapsed/refractory and newly diagnosed refractory multiple myeloma patients treated at our hospital between January 2011 and February 2015. The treatment was given in 28-day cycles and consisted of Len 15 mg/d for 21 days and a 7-day drug break. PRD 15 mg/d, 3 times/wk was administered for 4 weeks. The dosages of Len and PRD were increased or decreased as needed with discretion of the attending physician. All patients were enrolled in Rev-Mate which is patient’s registration system and strictly adhered to safety guidelines. Low-dose aspirin for thromboprophylaxis was recommended and the use of granulocyte colony stimulating factor (G-CSF) was implemented to adverse events such as neutropenia by the doctor’s judgment. Treatment
efficacy was assessed according to the International Uniform Response Criteria for Multiple Myeloma6 and adverse events by NCI-CTCAE Ver. 4. EZR was used for statistical analysis.
RESULTS
Patient Characteristics
Table 1 shows patient characteristics. Patients enrolled were 12 relapsed refractory and 7 newly diagnosed refractory patients, and 3 patients who switched to this modality due to adverse effects in past treatments. Fifteen patients had bone lesions. Four of them formed tumors. RP therapy was introduced after dexamethazone monotherapy (dex×20 mg×4 d) for newly diagnosed refractory patients. Pre-treatment consisted of a regimen including Bor or for 10 patients, and cyclophosphamide plus bortezomib plus dexamethasone (CBD) and bortezomib plus dexamethasone (Bd) regimens for 6 and 4 patients, respectively.
Table 1: Patient Characteristics.
|
Variable
|
n
|
%
|
| Age (years) |
|
|
| Median |
75.5
|
|
| rRange |
65-88
|
|
| >75 |
12
|
45.4
|
| Male(sex) |
10
|
45.5
|
| ISS |
|
|
| I |
8
|
36.3
|
| II |
6
|
27.2
|
| III |
7
|
31.8
|
| ND |
1
|
5
|
| Isotype |
|
|
| IgG |
14
|
63.6
|
| IgA |
4
|
18.1
|
| IgD |
1
|
0.5
|
| B-J |
2
|
9
|
| Plasmacytoma |
1
|
0.5
|
| PS |
|
|
| 1 |
7
|
31.8
|
| 2 |
6
|
27.3
|
| 3 |
7
|
31.8
|
| 4 |
2
|
9
|
| Creatinine |
|
|
| >2 mg/dl |
4
|
18
|
| Previous therapy |
|
|
| Bor base regimen |
10
|
45.5
|
| >3 regimen |
1
|
0.5
|
| ABMT |
1
|
0.5
|
| Only dex |
7
|
31.8
|
| Reason for change |
|
|
| PD |
11
|
50
|
| AE |
3
|
13
|
| QOL |
1
|
5
|
| Len induction time from diagnotis day |
|
|
| All cases |
932
|
|
| NDDM |
24
|
|
| RRMM |
1356
|
|
ISS: International Staging System; Bor: Bortezomib; ABMT: Autologous Bone Marrow Transplantation; PD: Progressive Disease; AE: Adverse Event; QOL: Quality of Life; Len: Lenalidomide; NDDM: Newly Diagnosed Multiple Myeloma; RRMM: Relapse and Refractory Multiple Myeloma.
Outcomes
Best treatment outcomes are shown in Table 2. Complete response (CR), very good partial response (VGPR) and partial response (PR) was founded in 3, 1 and 18 patients, respectively. Patients with a tumor lesion were 4 cases, but tumor regression was found in all patients. Efficiency equal to or superior to PR was found in patients aged ≥75 years, and treatment efficacy was found in very elderly patients as well. Among newly diagnosed refractory patients, complete response (CR), very good partial response (VGPR) and PR were seen in 1, 1 and 6 patients, respectively. The overall threeyear survival rate was 58% and time to the next treatment (TTNT) was 1 year in 49% of patients. As such, despite responding to the treatment, half of the patients required the next treatment in one year (Figure 1). The reasons for changing treatment modalities included progressive disease, adverse effects, and by patient choice in 9, 3 and 7 patients. Treatment outcomes, age, International staging system stage, new diagnosis, past history of Bor use or adverse effects did not result in significantly different survival rate. Outcomes were significantly poor prognosis in patients with renal failure. (p=0.035), The patients who could continue treatment for one year had significantly better outcomes (Figures 2 and 3).
Figure 1. Left: Kaplan-Meier Survival Analysis of OS. Right: Kaplan-Meier Survival Curves for TTNT
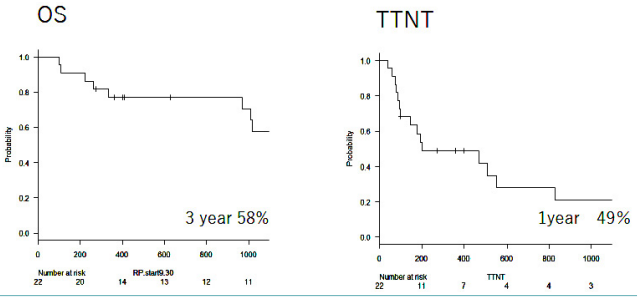
OS: Overall Survival; TTNT: Time to next treatment
Figure 2: Kaplan-Meier Survival Analysis of OS for Response: >VGPR versus <VGPR, >CR versus <CR. age: >75 versus <75,
ISS >2 versus <2, initial refractory and Pre Bor.
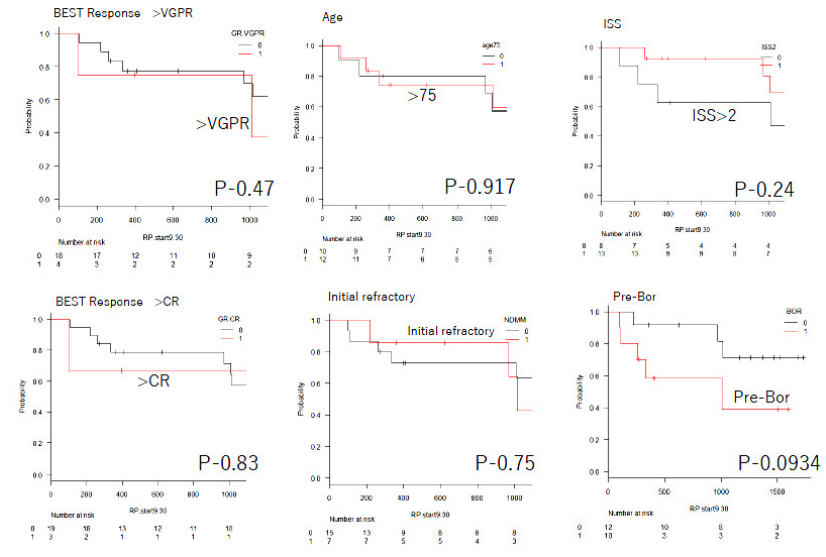
VGPR: Very Good Partial Response; ISS: International Staging System; Bor: Bortezomib; ABMT: Autologous Bone Marrow Transplantation; PD: Progressive Disease; AE: Adverse Event; Len: Lenalidomide; NDDM: Newly Diagnosed Multiple Myeloma; RRMM: Relapse and Refractory Multiple Myeloma.
Figure 3: Kaplan-Meier Survival Analysis of OS for Adverse Events: >G3 versus <G3, Rash,
Renal Failure and Continue Treatment.
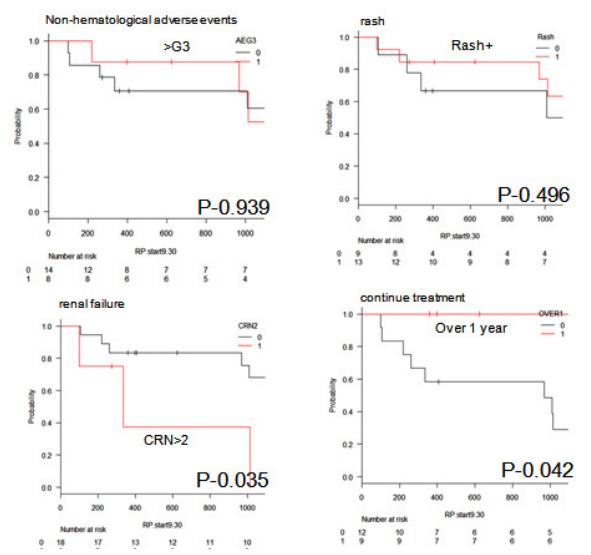
OS: Overall Survival; CRN: Creatinine; RP: Prednisolone.
Table 2: Outcomes.
|
Best response
|
n
|
%
|
| CR |
3
|
14
|
| VGPR |
1
|
4
|
| PR |
18
|
82
|
| Best Response for >75 years |
|
|
| CR |
1
|
8
|
| VGPR |
0
|
|
| PR |
11
|
92
|
Safety
Hematological adverse events are shown in Table 3. They were leukopenia (G1-2 23%, G3 9%), anemia (32% G1-2 and 18% G3) and thrombocytopenia (14% G1-2 and 9% G3). Non-hematological adverse events included rash (41% G1-2 and 23% G3), peripheral nerve disorder (23% G1-2, none above G3), and G3-4 renal failure in 10%. Infections of G3 occurred in 5%, and there was no deepvein thrombosis. Len dose was reduced in 7 out of 22 patients for lowered leukocyte count (2 patients), rash (4 patients) and abdominal pain (1 patient). There were 6 patients that required interruption of treatment for adverse effects of peripheral nerve disorder, rash, elevated CRP, fatigue and complication in 1, 3, 2, 1 and 1 patients, respectively, including patients with multiple adverse effects requiring interruption.
Table 3: Adverse Events.
|
Adverse event (%)
|
G1-2 (%)
|
G3 (%)
|
G4 (%)
|
| Hematological |
|
|
|
| Neutropenia |
23
|
9
|
0
|
| Anemia |
32
|
18
|
0
|
| Thrombocytopenia |
14
|
9
|
0
|
| Non-Hematological |
|
|
|
| Rash |
41
|
23
|
0
|
| Infection (pneumoniae) |
0
|
5
|
0
|
| TLS |
0
|
9
|
0
|
| Edema |
5
|
0
|
0
|
| Diarrhea |
5
|
5
|
0
|
| Peripheral neuropathy |
23
|
0
|
0
|
| Fatigue |
9
|
0
|
0
|
| Elevation CRP |
0
|
0
|
14
|
| Creatinine increase |
0
|
5
|
5
|
DISCUSSION
In the present research, we proved that RP therapy on Japanese elderly multiple myeloma patients was safe and effective. The mean dosage was Len 12.5 mg/d and PRD 7.9 mg/d. The dosage of PRD was extremely low compared to RP therapy reported abroad. Despite this, we found efficiency superior to PR in all patients. Three-year survival rate was 58% (Figure 1). Although, there was no difference in survival rate between differences of ISS, history of PS or Bor use or newly diagnosed refractory multiple myeloma, the results suggested a possible lower survival rate in patients with history of Bor pre-treatment (p=0.09). For patients using Bor previously, We think it is necessary to adjust the dosage of Len dosage such as increasing dosage. The therapeutic effect of RP was poor in patients with renal failure. This may be caused by either exacerbated symptoms, or because the renal failure itself had an impact on general state of elderly patients thus impacting the prognosis. However, the number of cases with this adverse effect was few in our study and Len is reported to be effective even with renal failure, thus there is a need to investigate further based on more data on future patients.7,8
Furthermore, the appearance of skin eruption has been reported to have a good prognosis. But we did not find a significance difference through our analysis.9 This is believed to be due to the high number of patients that stopped treatment because of rash. It may be important to continue Len by making some adjustments in rash management. Although, the difference was not significant, patients with rash onset had higher doses of Len; thus, reducing the Len dose may be an option. Also, elderly patients are more prone to losing motivation to continue the treatment when they have rash, so it may be equally important to thoroughly educate patients about rash. Considering that the prognosis is good in patients who could continue the treatment, it is possible that management of adverse effects largely contributes to the treatment outcomes in elderly patients. However, in this study, TTNT was 49% in one year. It was hard to maintain long-term remission. The most common reason for prescription change was disease progression, but as much as 36% of changes were made due to patient’s intention. Some cases seems to have been able to be continued Len in the discontinued cases, so it may require some tips on the side of the doctor as well. As shown in Table 4, hematotoxicity was low compared to other reports in terms of adverse effects, although the difference in sample size must be noted. The low number of patients with leukopenia and low dosages of steroid led to a decrease in the incidence of infections.
Table 4: Summary of Side Effects of G3 or more in Clinical Trials in Multiple Myeloma.
|
Trial
|
VISTA |
FIRST |
FIRST |
MM009/10 |
|
|
|
G3 (%)
|
VMP |
Rd Continous |
MPT |
Rd |
RP |
RP (This study) |
| Neutropenia
Anemia
Thrombocytopenia |
40
19
37
|
28
18
8
|
45
19
11
|
35
11
14
|
22
11
2
|
9
18
9
|
| Infection
Rash
Fatigue
PN
DVT |
10
1
8
13
1
|
29
6
7
1
8
|
17
5
6
9
5
|
19
–
7
1.7
12
|
11
17
2
4
–
|
5
23
0
0
0
|
Management of side effects is important from the point of treatment of elderly patients as well. Regarding non-hematological toxicity, incidence of rash was higher compared to other reports, most likely as a result of the low doses of the steroid. Some patients refused RP therapy due to anxiety about rash onset; thus, determining the optimal dose is essential. Thrombosis is an important adverse effect of Len, but did not occur in this study. Some elderly patients have declined performance status and it may be difficult to go to hospital, so it is very useful that treatment can be given by oral pharmacotherapy. Thirty-one percentage of patients required dose reduction of Len. Considering their advanced age, initial dose should be 5-10 mg/d, and increased gradually while monitoring for adverse effects. In examples of renal dysfunction, it is important to watch out for blood toxicity, but in this study, relative safe administration was possible in patients with renal dysfunction. Other adverse events included increase in C-reactive protein in a few patients. Since no clear symptoms of infection were found despite full body examinations, it is likely due to the immune mechanisms of Len and possibly related to the dose of prednisolone (PSL), which was low. In conclusion, Len and low-dose PLD can be safely and effectively administered in elderly patients.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest. This study has passed through the Institutional Review Board (IRB) of our hospital.








