INTRODUCTION
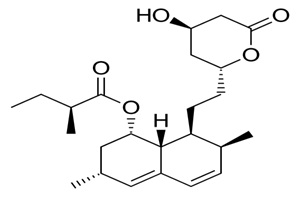
Among the most commonly prescribed drugs worldwide, are cholesterol-lowering agents statins – used to manage cardiovascular and other related heart diseases.1 Even though statins have been proven to significantly reduce the risk of cardiovascular disease (CVD) and its associated mortality,2 statins therapy may lead to an increased risk of type II diabetes (Figure 1).3
Figure 1. Structural Analogue of Statins (Simvastatin)
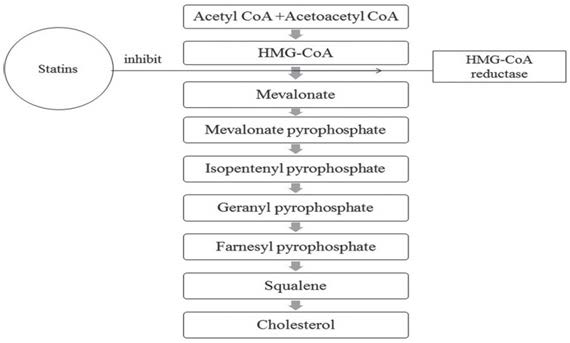
Statins function by controlling the metabolic pathway at the level of β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase in producing cholesterol. HMG-CoA reductase is the rate-controlling enzyme in the mevalonate pathway, which is the sole contributor to the production of cholesterol. As a result, it is the target for pharmacokinetics to utilize inhibitors to reduce the production of cholesterols while increasing the expression of low-density lipoproteins (LDL) receptors.4
The binding of statins to the mammalian HMG-CoA reductase at lower concentrations causes a displacement effect of the substrate HMG-CoA, which then binds at a concentration of micromolar.5 Interactions between HMG-CoA reductase and statins inhibit the conversion of HMG-CoA to L-mevalonate, leading to the inhibition of down stream cholesterol biosynthesis and numerous metabolites such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP1) (Figure 2).
Figure 2. Inhibition of Cholesterol Biosynthesis Pathway by Statins
PATHOPHYSIOLOGY AND ETIOLOGY OF TYPE II DIABETES
Type II diabetes is triggered by a mix of genetic factors associated with compromised insulin secretion, resistance, and environmental factors including; obesity, stress, overeating, lack of exercise, as well as aging. It has also been reported that Japanese individuals may have many diabetes-sensitive genes including thrifty genes. The number of diabetic patients is rapidly increasing, reflecting the changes in lifestyle.6
STATINS AND TYPE II DIABETES
Though it is well-documented that statins considerably decrease the risk of developing CVD. Nonetheless, statin chemotherapy could lead to an increased risk of type II diabetes.2 Current theories of type II diabetes mellitus include a defect in insulin-mediated glucose uptake in muscle, a dysfunction of the pancreatic β-cells, a disruption of secretory function of adipocytes, and impaired insulin action in the liver.3
PRECISE MECHANISMS OF DIABETOGENICITY
Statins-induced diabetes remainsun certain; meanwhile, there have been several proposed mechanisms, most of which fall within two distinctive domains (Figure 3). The major mechanism associated with statin-induced diabetes is β-cell dysfunction, which included altered insulin secretion and sensitivity, changes in ion channel conductance, and oxidative stress and inflammation.
Figure 3. Mechanism of Statins- Induced Type II Diabetes

Statins may also induce diabetes via the dysregulation of insulin secretion endogenously. The major signal for the release of insulin from the pancreatic β-cell is glucose. Glucose is taken up into the pancreatic β-cells via glucose transporter 2 (GLUT2), where it is phosphorylated to glucose-6-phosphate (G-6-P) by either the glucokinase or hexokinase IV enzyme. Subsequently, the glycolytic pathway is activated, leading to increased production of adenosine triphosphate (ATP), which later closes the ATP-gated potassium ion channels resulting in membrane depolarization.
Depolarized membrane promotes the influx of calcium ion through L-type calcium channels, induce exocytosis of insulin granules. Statins, on the other hand, inhibit voltage-gated calcium channels in the β-cells of the pancreas resulting in decreased insulin secretion and insulin retaining granules exocytosis.7 Rosuvastatin has been shown to have an inhibitory effect on glucose-induced insulin secretion through the inhibition of HMG-CoA reductase, rather than the calcium channels of the pancreas, as this action can be reversed by mevalonic acid.
It has been shown that specific statin drug, rosuvastatin influence the secretion of insulin via the same pathway it affects the cholesterol levels. Also, the levels of coenzyme Q10 can be reduced by statins. CoQ10 is a constituent of the electron transport chain involved in the generation of ATP.8 Delayed production of ATP can be a result from reduced levels of CoQ10 and subsequently, diminished release of insulin. Pancreatic β-cell mitochondrial dysfunction, skeletal muscle cells, and a9dipocytes have been implicated in the pathogenesis of diabetes.10,11,12 Aside from reduced secretion of insulin from pancreatic β-cells, noteworthy evidence proposed that insulin sensitivity can be altered with statin-use and precipitate widespread insulin resistance. Therefore, it was assumed that diabeto genesis might be related to the consequence of statins on insulin sensitivity in the liver and muscle.13 Inhibition of isoprenoid biosynthesis by statins has been implicated in the downregulation of glucose transporter (GLUT4), which mediates glucose uptake in the adipocytes.14 Down regulation of GLUT4 results in enhanced resistance to insulin in adipocytes, muscle, and liver.
The metabolism of carbohydrates and fatty acids in liver and muscle influences insulin resistance by reducing hepatic gluconeogenesis and upregulating glucose uptake and beta-oxidation in the muscle, which is affected by the adiponectin released from the adipocytes. Therefore, insulin resistance has been shown to have a pathophysiological effection type II diabetes development.
Although, statins have been shown to have a beneficial effect in reducing the susceptibility to cardiovascular events, however, its glycemic implication on patients should be examined frequently by evaluating the glycemic index irrespective of the patient developing diabetes or not. Further studies are important to determine whether or not the dose and duration of statins use could affect glycemic control.
STATINS TOXICITY
Statin is not synthesized in the animal or human body. Specific statins such as mevastatin, lovastatin, and pravastatin are natural statins drug of fungal origin,and simvastatin is a semi-synthetic drug derived from lovastatin. Meanwhile, fully synthetic satins are atorvastatin, cerivastatin, fluvastatin, pitavastatin, and rosuvastatin.15,16 The general safety of statins is widely accepted, but there have been reports of adverse effects resulting in altered renal, hepatic, and musculature function.17
The most common adverse effects of statins are on the skeletal muscle, which ranges from a mild myopathy such as cramp, fatigability, and exercise intolerance to myotoxicity. In rare cases, severe rhabdomyolysis may develop.18 Rhabdomyolysis is a complex health condition characterized by swift dissolution of damaged muscle with increment in the release of toxic intracellular muscle components. Simvastatin also induces apoptosis in cardiac myocytes,19 human skeletal muscle cells,20 T-cells, B-cells, and myeloma tumor cells.21 Other possible toxicological effects of statin therapy include-hemorrhagic stroke, depression, and lower test osterone, decreased renal function, tendon rupture, elevated liver function tests, interstitial lung disease, among others.22
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








