INTRODUCTION
Fatigue can be categorized into two types: physical fatigue (which affects the muscles) and mental fatigue (which impacts the nervous system), depending on its underlying mechanism. Physical fatigue is accompanied by a decrease in muscle function, a sense of tiredness, and an inability to sustain the original level of activity.1 Factors such as an imbalanced diet, high life stress, or irregular sleep patterns exacerbate fatigue symptoms.2 The causes of fatigue are complex and diverse. First, prolonged muscle exercise accumulates energy metabolites such as lactate or blood urea nitrogen (BUN). Second, the production of large amounts of reactive oxygen species causes an increase in oxidative stress, leading to organ damage that is harmful to health.3 In addition, insufficient glycogen storage in the liver and muscles and imbalanced blood-oxygen concentration reduce the recovery ability of the body and affect exercise performance.4 Short-term fatigue could be improved through enough rest, however, lasting fatigue (>6-months) leads to chronic fatigue, which decreases immune system function and may cause diseases such as systemic lupus erythematosus, rheumatoid arthritis, and sleeping dysfunction.5 Previous research has shown that functional material supplementation such as flavonoids, helps reduce the accumulation of energy metabolites and improve energy utilization.6 However, there have been limited studies on the anti-fatigue effects of combined herbal supplements. Therefore, it is worthwhile to develop a functional herbal supplement that could help alleviate fatigue.
Rhodiola rosea (RHR), known as ‘roseroot’ or ‘golden root’, belongs to the Crassulaceae family and is native to the Qinghai-Tibet Plateau region. RHR contains more than 140 different compounds, including flavones, anthraquinones, coumarins, volatiles, and organic acids. Unique to plants in the Rhodiola genus, it contains salidroside (rosavin, rosin, rosarin), which imparts various beneficial effects, including anti-diabetic, anti-aging, anti-cancer, neuro-protective, and cardio-protective properties.7 Studies have shown that supplementation with salidroside enhanced the swimming endurance and forelimb grip strength of mice, reduced the accumulation of fatigue-inducing substances, and exhibited an anti-fatigue effect.8 Maca (Lepidium meyenii) is native to the Andes region, growing at altitudes between 3,500 and 4,450 meters. It is rich in water-soluble polysaccharides, endowing it with antioxidant and immune-modulating properties. Maca has also been shown to enhance the exhaustive swimming time of mice and reduce levels of lactate and BUN, demonstrating its anti-fatigue effects.9 Fenugreek (Trigonella foenum-graecum), a member of the Leguminosae family, is a plant considered both a food crop and a medicinal herb in China and North Africa. Research has shown that fenugreek extract can regulate the levels of total antioxidant capacity (TAC), malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), and nitric oxide, exerting antioxidant effects that contribute to the reduction of skeletal muscle fatigue.10 Cistanche tubulosa (family Orobanchaceae), containing eight phenylethanoid glycosides, ten iridoid glycosides, syringalide A 3-O-α-L-rhamnopyranoside, phenylethyl oligosaccharides, terpenes, lignans, and other active compounds, has been studied for its potential health benefits. Research has demonstrated that supplementation with Cistanche tubulosa extract can extend the survival time of mice under hypoxic conditions.11 Another study has shown that Cistanche tubulosa extract has the potential to prolong swimming time in mice, indicating its antifatigue properties.12 Velvet antler is the only organ among mammalian species capable of permanent regeneration. Its regenerative capacity is associated with the stem cells within velvet antlers which facilitate wound healing.13 Furthermore, arginine and vitamin B are essential nutrients for the body and have been proven to contribute to the recovery from fatigue.14,15
A previous study has indicated that a supplement combining RHR with blueberry extract, green tea extract, vitamin C, and vitamin D could help maintain energy balance during exercise and reduce post-exercise fatigue, demonstrating its anti-fatigue efficacy caused by exercise.16 It is evident that the phytochemicals rich in herbal plants have the potential for antioxidant, anti-fatigue, and anti-inflammatory effects, and combinations might exhibit synergistic effects. Therefore, this study employed a mouse exhaustive swimming test to assess the effect of an RHR supplement (combined with the extract of Lepidium meyenii, Trigonella foenumgraecum and Cistanche tubulosa, velvet antler powder, L-arginine, and vitamin B) in extending endurance during exercise and reducing the accumulation of fatigue relative substances.
METHODOLOGY
Experimental Supplement
The RHR supplement was provided by HealthTake Co., Ltd. (731.6 mg per tablet). The formula contained Rhodiola rosea extract combined with herbal extract (Lepidium meyenii, Trigonella foenum-graecum and Cistanche tubulosa), velvet antler powder, L-arginine, and vitamin B.
Study Design
Forty institute of cancer research (ICR) mice (6-week-old males) were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). All animals were housed under standard conditions (temperature 22±2 °C, humidity at 60-70%, and 12-h light/12-h dark cycle), and were provided a standard laboratory diet (No. 5001; PMI Nutrition International, Brentwood, MO, USA) and distilled water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the National Taiwan Sport University (IACUC-11013). After two weeks of adaption, the mice were randomly assigned to one of four groups (n=10): the control group received distilled water, and the RHR supplement groups received 1X, 2X, and 5X RHR supplement (600, 1200, and 3000 mg/ kg mouse). All mice received samples with RHR by oral gavage for five consecutive weeks, and body weight, water consumption, and feed intake were recorded each week.
Exhaustive Swimming Test
The exhaustive swimming test was performed after treatment for 28 days (day 29). The protocol was referred to the method of Wu et al.17 After 30-minutes of intervention, the mice with 12-hours diet deprivation were loaded with 5% of their body weight on the tails, placed individually in a columnar swimming pool (14 cm radius×25 cm deep water being maintained at 27±1 °C), and forced to swim. The swimming endurance of each mouse was determined from when the head of the mice fell under the surface of the water for 8-seconds, and the time from the beginning of swimming to exhaustion was recorded.
Blood Fatigue Biomarkers Analysis
To evaluate the effect of the RHR supplement on fatigue biomarkers, the mice performed two swimming tests on day 31 (swimming for 10-minutes) and day 33 (swimming for 90-minutes).18 All mice were fasted for 12-hours before two tests. On day 31, after a minute intervention, the mice were forced to swim for 10 minutes at 30 °C. Blood samples were collected for lactate analysis before swimming (baseline), immediately after swimming, and after a 20-minute rest. On day 33, after 30-minutes of intervention, the mice were forced to swim for 90-minutes at 30 °C following 60-minutes of rest, and the blood was collected to determine the serum level of BUN and creatine kinase (CK). Serum was obtained after centrifugation (1500 g, 15 minutes, 4 °C), and the fatigue biomarkers were measured with an automatic analyzer (model 7060, Hitachi, Tokyo, Japan).
Hepatic and Muscle Glycogen Analysis
The mice were sacrificed 30 minutes after the last intervention and the liver and muscle were collected and weighed for tissue glycogen level analysis. The glycogen content assay was performed according to the method of Huang et al6 100 mg of liver and muscle tissue were homogenized in 0.5 mL of cold perchloric acid following centrifugation at 12,000 ×g for 15-minutes at 4 °C. The supernatant was then transferred into a 96-well plate (30 μL), and 200 μL of iodine-potassium iodide reagent was added to each well to facilitate iodine binding to glycogen. After 10-minutes, the absorbance was measured at a wavelength of 460 nm using an ELISA reader (Tecan Infinite M200, Tecan Austria, Austria). The values of glycogen in the analyzed samples were calculated using a standard curve of glycogen (Sigma) and expressed as milligrams of glycogen per gram wet weight of tissue.
Statistical Analysis
Results were expressed as the mean±SD. All experimental data were performed by one-way analysis of variance (ANOVA) with Duncan’s post hoc test, and a significant difference between groups was determined to be p<0.05. All statistical analyses were performed with Statistical Analysis System (SAS) version 9.0 statistics software (SAS Institute, Cary, NC, USA).
RESULTS
Effect of RHR Supplement on the Growth Parameters of ICR Mice
Table 1 shows the condition of mice after 5 weeks of RHR supplementation. A results (Table 1), there was no significant difference in the final body weight, feed intake, energy intake (from feed, supplement, or total energy), and water intake among in each group.
| Table 1. Effect of Rhodiola Rosea (RHR) on Growth Parameters |
| Growth Parameters |
Control |
RHR |
| 1X |
2X |
5X |
| Initial body weight (g) |
33.6±1.2 |
33.6±0.7 |
33.1±1.2 |
33.6±0.9 |
| Final body weight (g) |
38.0±1.3 |
38.0±1.3 |
37.9±0.9 |
38.0±0.7 |
| Feed intake (g/mice/day) |
6.5±0.7 |
6.5±0.8 |
6.5±0.8 |
6.5±0.8 |
| Energy intake from feed (kcal/mice/day) |
21.8±2.5 |
21.8±2.5 |
21.8±2.7 |
21.8±2.6 |
| Energy intake from supplement (kcal/mice/day |
0.00±0.00 |
0.08±0.00 |
0.15±0.01 |
0.39±0.02 |
| Total energy intake (kcal/mice/day) |
21.8±2.5 |
21.8±2.5 |
21.9±2.7 |
22.2±2.6 |
| Water intake (mL/mice/day) |
7.0±0.2 |
7.0±0.2 |
7.0±0.1 |
7.0±0.3 |
| Total energy intake (kcal/mice/day)=energy intake from feed (kcal/mice/day)+energy intake from supplement (kcal/mice/day). The reported values are the mean±SD (n=10). There is no significant difference of above data between the groups. |
Effect of RHR Supplement on Exercise Endurance of ICR Mice
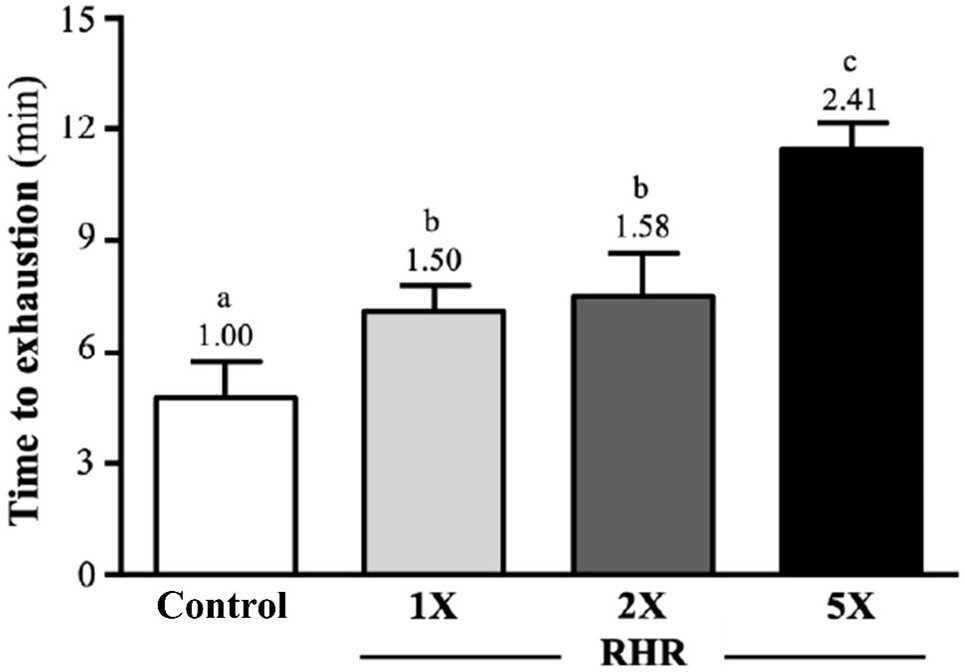
The results of exhaustive swimming time of mice after 4-weeks of RHR supplementation are shown in Figure 1. The time to exhaustion in the control, RHR-1X, RHR-2X, and RHR-5X groups was 4.74±0.98, 7.12±0.68, 7.51±1.16, and 11.44±0.72-minutes. Compared with the control group, the RHR-1X, RHR-2X, and RHR5X groups were significantly greater by 1.50-fold, 1.58-fold, and 2.41-fold p<0.0001) respectively.
Figure 1. Effect of Rhodiola Rosea on Exhaustive Swimming Time of ICR Mice
The reported values are the mean±SD (n=10). Different superscript letters (a, b, and c) indicate significant difference between groups (p<0.05)
Effect of RHR Supplement on Serum Lactate Level after a 10-minute Swimming Test
The 10-minute swimming test was carried out to evaluating the change of blood lactate levels after 4-weeks of RHR supplementation. As shown in Table 2, there was no significant difference between groups before swimming p>0.05). The level of lactate increased after 10-minutes of swimming, and decreased after 20 minutes of rest. The RHR treatment groups (1X, 2X, and 5X) had lower lactate level after swimming when compared to the control group (reduced by 21.71%, 27.55%, and 31.29%, respectively), and showed a significant difference p<0.0001). The lactate production rate in RHR treatment groups (1X, 2X, and 5X) were significantly reduced by 22.65, 29.49%, and 33.33%, respectively when compared to the control group (p<0.0001).
| Table 2. Effect of Rhodiola Rosea (RHR) on the Level of Serum Lactate after a 10-minute Swimming Test |
|
Groups
|
Control |
RHR |
| 1X |
2X |
5X
|
| Lactate level (mmol/L) |
|
Before swimming (A
|
2.90±0.50a |
2.90±0.33a |
2.93±0.31a |
2.94±0.29a |
| After swimming (B) |
6.68±0.32c |
5.23±0.57b |
4.84±0.54ab |
4.59±0.51a
|
|
After a 20-minute rest (C)
|
5.46±0.44c |
4.14±0.56b |
3.72±0.4a |
3.33±0.33a
|
| Change rate (fold) |
|
Clearance rate=(B-C)/B
|
2.34±0.28c |
1.81±0.06b |
1.65±0.09a |
1.56±0.09a |
| Clearance rate=(B-C)/B |
0.18±0.03a |
0.21±0.04ab |
0.23±0.04b |
0.27±0.03c
|
| The rate of lactate production rate (fold)=(the value after swimming (B)–the value after a 20- minute rest (C))÷the value after a 20-minute rest (A). The rate of lactate clearance (fold)=the value after swimming (B)÷the value after a 20-minute rest (A). The reported values are the mean±SD (n=10). There is no significant difference of above data between the groups. Different superscript letters (a, b and c) indicate significant difference between groups (p<0.05). |
After 20-minutes of rest, RHR supplementation groups (1X, 2X and 5X) showed lower values of serum lactate (reduced by 24.18%, 31.87% and 39.01%, respectively), and there was a significant difference p<0.0001). In addition, the clearance rate of lactate was significantly higher in the group of 2X and 5X RHR supplementation groups by 1.28-fold (p=0.0077) and 1.50-fold (p<0.0001) respectively than the control group.
Effect of RHR Supplement on Serum CK and BUN Level after a 90-minute Swimming Test
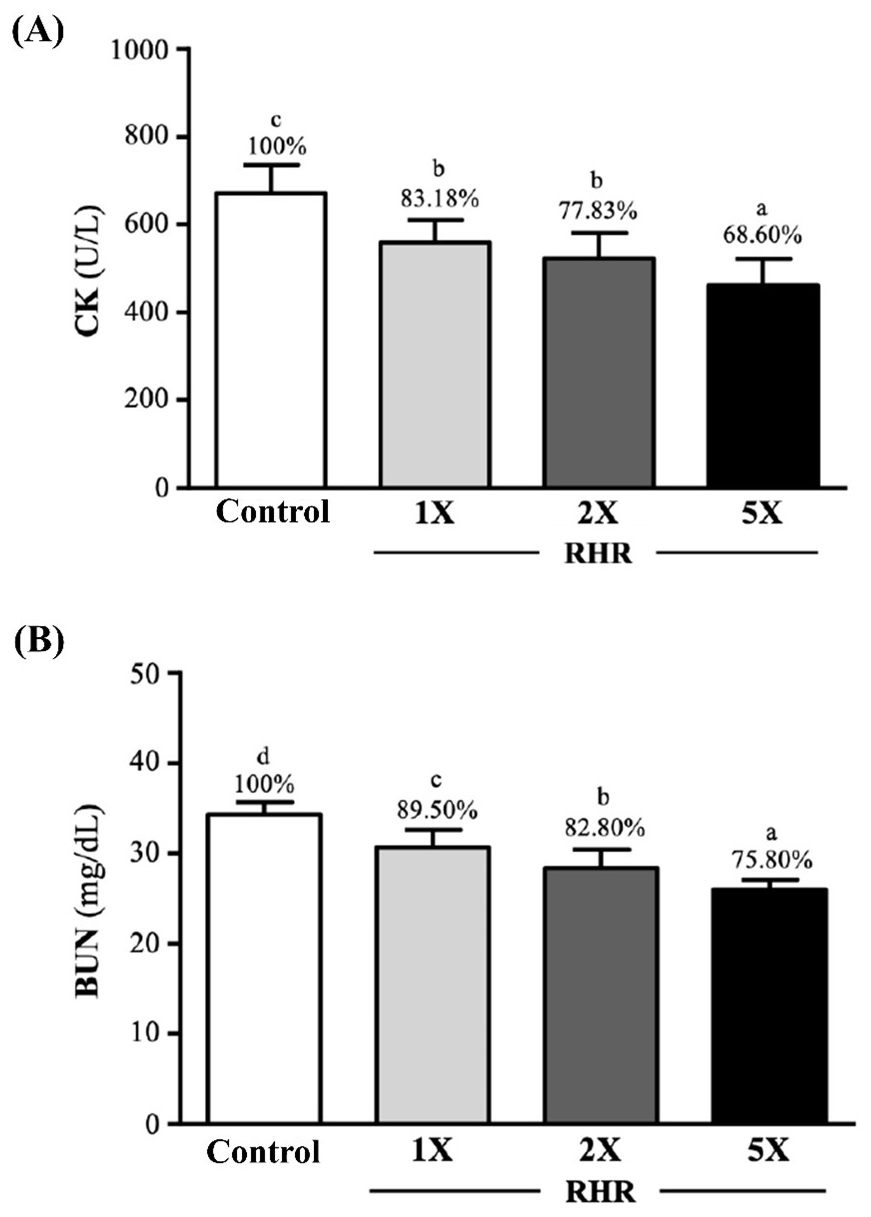
The results of serum CK (A) and BUN (B) level after 90-minutes swimming exercise following 60-minutes rest are shown in Figure 2. The CK activity in the control, RHR-1X, RHR-2X and RHR5X groups was 672±64, 559±51, 523±57, and 461±61 (U/L), respectively. The RHR supplementation groups (1X, 2X and 5X) significantly reduced the CK activity by 16.82% (p=0.0001), 22.17% (p<0.0001) and 31.40% (p<0.0001) when compared to the control group. The BUN level in the control, RHR-1X, RHR-2X, and RHR-5X groups was 34.4±1.4, 30.7±1.9, 28.4±2.0, and 26.0±1.1 (mg/dL), respectively, and the RHR supplementation groups (1X, 2X and 5X) significantly reduced the BUN level by 10.50% (p<0.0001), 17.20% (p<0.0001), and 24.20% (p<0.0001).
Figure 2. Effect of Rhodiola Rosea on the Level of Serum CK (A) and BUN (B) of ICR Mice after a 90-minute Swimming Exercise and 60-minute Test
The reported values are the mean±SD (n=10). Different superscript letters (a, b, c, and d) indicate significant difference between groups (p<0.05). CK, creatine kinase. BUN, blood urea nitrogen.
Effect of RHR Supplement on Tissue Glycogen Storage
The hepatic glycogen (A) and muscle glycogen (B) content are presented in Figure 3. The hepatic glycogen content in the control, RHR-1X, RHR-2X and RHR-5X groups was 20.35±3.61, 29.62±4.15, 35.51±5.66 and 38.12±5.82 (mg/g liver), respectively.
Figure 3. Effect of Rhodiola Rosea on the Level of Hepatic Glycogen (A) and Muscle Glycogen (B) of ICR Mice
The reported values are the mean±SD (n=10). Different superscript letters (a, b and c) indicate significant difference between groups (p<0.05).
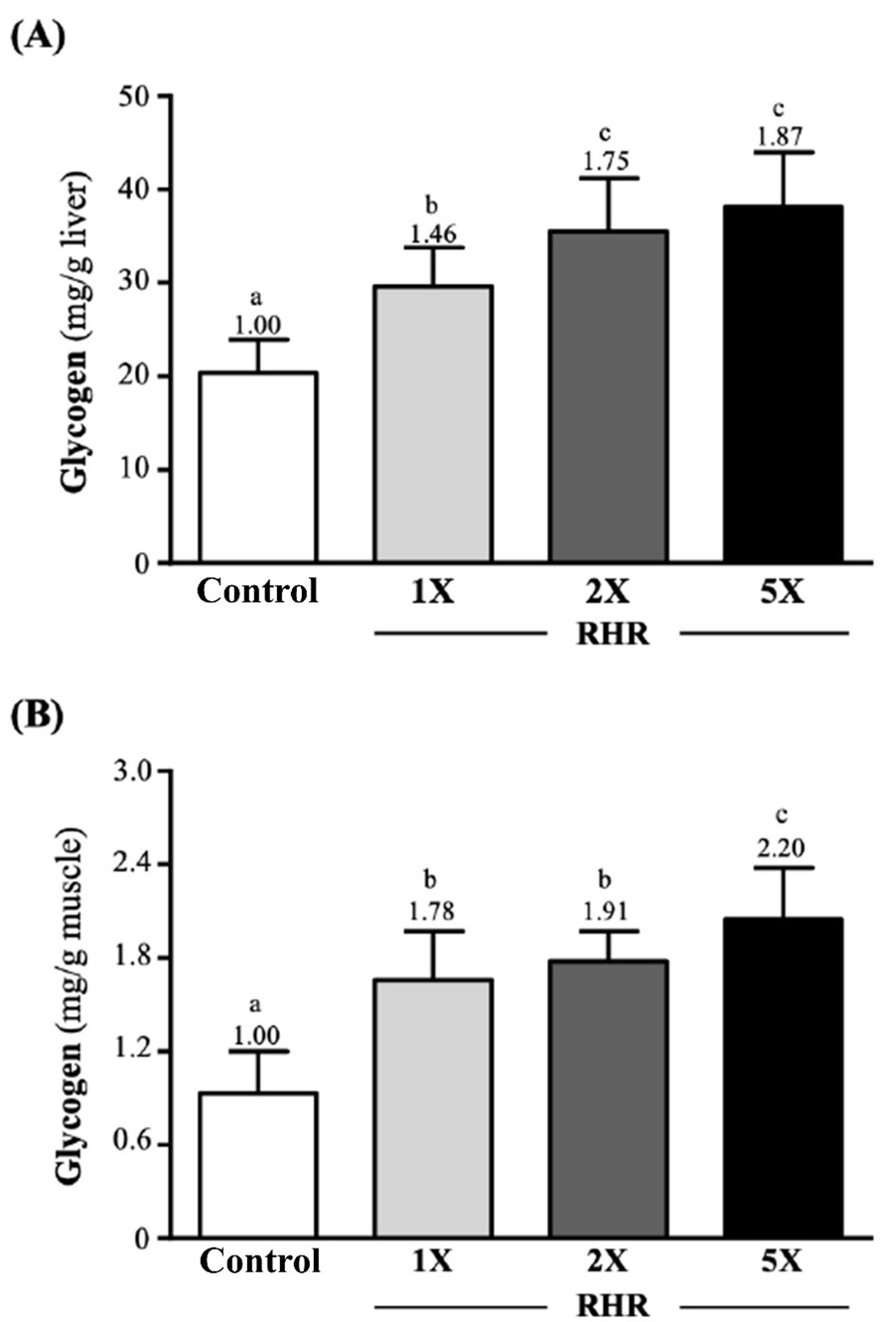
The RHR supplementation groups (1X, 2X and 5X) significantly increased the hepatic glycogen content by 1.46-fold (p=0.0002), 1.75-fold (p<0.0001), and 1.87-fold (p<0.0001) when compared to the control group. The muscle glycogen content in the control, RHR-1X, RHR-2X, and RHR-5X groups was 0.93±0.27, 1.66±0.31, 1.78±0.19, and 2.05±0.33 (mg/g muscle), respectively. The RHR supplementation groups (1X, 2X and 5X) significantly increased the muscle glycogen content by 1.78-fold (p<0.0001), 1.91-fold (p<0.0001), and 2.20-fold (p<0.0001) when compared to the control group.
DISCUSSION
Fatigue is a phenomenon resulting from an imbalance between energy supply and demand, and excessive physical activity, leading to the accumulation of metabolites in the blood (such as lactate, CK, and BUN), as well as glycogen depletion in the liver and muscle.15 Previous studies have shown that plants rich in active compounds, such as polyphenols or glycosides, possessed strong antioxidant properties, and improved energy metabolism, which had anti-fatigue effects.10,19,20 For example, myrtenol and geraniol, present in RHR, exhibit excellent antioxidant and anti-inflammatory properties, having the ability to reduce the free radicals and inflammatory responses induced by exercise and, therefore, mitigate adverse effects resulting from excessive physical activity.21 In this study, an RHR supplement was administered to mice for a 5 week intervention at doses of 1, 2, and 5 times, followed by a swimming test. The results of this study indicate that supplementation with the RHR supplement for 5-weeks did not significantly affect growth parameters such as body weight, energy intake, or water intake in mice (Table 1). Although the body weights of all four groups increased by 13%, it was consistent with normal growth patterns, and the ratio of increase remained within the range of typical growth in male ICR mice 6 to 12-weeks old (30 to 40 g), unaffected by the intervention.22,23 Additionally, the RHR supplement did not change the weight of organs, muscle tissue, and adipose tissue in mice (data not shown), and there was no animal with low activity, low appetite, or death during this study. It indicated that supplementation with the RHR supplement was not harmful to the growth of mice. Results of the present study were consistent with a study recruiting 100 male and female participants with prolonged chronic fatigue symptoms, in which supplementation with RHR extract for 8-weeks led to a significant improvement in fatigue symptoms without observed adverse effects.24 These results suggested that RHR might be a safe functional material, and was suitable for development into a dietary supplement for anti-fatigue.
During high-intensity voluntary exercise, the occurrence of tissue hypoxia and a reduction in femoral venous oxygen saturation are recognized to coincide with the production of lactate. While low-intensity exercise also results in the production of lactate in fast-twitch glycolytic muscle fiber.25 The accumulation of lactate in both the blood and muscles lowers the pH level and leads to fatigue. Thus, blood lactate concentration is often used as an indicator for assessing exercise intensity and fatigue level.4 Previous studies have shown that the supplementation of 100 mg/kg of RHR extract given to mice for 20-days significantly enhanced the exhaustion time of the weight-loading swimming test. Additionally, a 30-day supplementation significantly reduced blood lactate levels after swimming exercise, demonstrating the anti-fatigue function of RHR.26 Another study demonstrated that mice supplemented with 1, 2 and 4 grams of Maca extract for 30-days significantly improved forelimb grip strength and rota-rod performance compared to the control group, along with lower blood lactate levels after exercise.27 In this study, mice supplemented with RHR supplements had significantly lower lactate levels after 10-minutes of swimming exercise and 20-minutes after exercise and had a lower lactate production rate as well as a higher lactate clearance rate (Table 2). It suggests that RHR supplementation might prevent fatigue occurrence and prolong exercise endurance by reducing lactate production and increasing lactate clearance.
When the body engages in prolonged physical activity and the energy supplied by carbohydrates and fats are insufficient, proteins and amino acids start to degrade, leading to the production of the end-product, BUN.28 Strenuous exercise also results in damage to muscle, increasing cell permeability and causing the release of CK into the blood.29 Thus, BUN and CK are commonly used as indicators to assess protein degradation and muscle damage. Our results showed that mice supplemented with RHR supplements for 33-days had significantly lower-levels of BUN and CK than the control group (Figure 2). A previous study has demonstrated that mice supplemented with RHR extract for 15-days (1.5 g/kg body weight) exhibited a significant increase in exhaustive swimming time, an elevation in the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), along with a reduction in blood BUN level post-exercise, which indicated the antioxidative and anti-fatigue effects of RHR extraction.30 In addition, a combined herbal extract compound (Withania somnifera, Silybum marianum and Trigonella foenum-graecum) increased the phosphorylation of Akt and p38 MAPK in C2C12 myotube, promoting protein synthesis and differentiation of myoblast, which decreased the degradation of muscle protein, and demonstrated a muscle protection effect.31 According to these studies, the anti-fatigue effects of RHR supplementation, might be related to its muscle-protective property.
Glycogen is one of the forms of stored energy within the bodies of animals, including hepatic glycogen and muscle glycogen. During endurance exercise, glycogen is decomposed to glucose to sustain energy expenditure during the activity. Therefore, when the glycogen content in the body is insufficient, it leads to fatigue and decreases exercise performance.32 Study has shown that in a low-oxygen environment, mice supplemented with RHR extract significantly prolonged the exhaustive swimming time and exhibited higher hepatic glycogen accumulation and ATP transporter enzyme activity, which reduced the consumption of energy substances and promoted the transport of energy substances.33 Additionally, RHR not only extended the time to exhaustion in mice but also enhanced glycogen synthesis in HepG2 cells by activating the AMPK pathway, leading to a significantly increased glycogen storage.34 The results of this study showed that supplementation with RHR supplements significantly increased exhaustive swimming time and increased the storage of muscle and hepatic glycogen after exercise (Figures 1 and 3). It suggested that the anti-fatigue effects of RHR supplementation might be related to its stabilization of energy supply during exercise.
Previous studies have shown that RHR increased the mobilization of triglyceride during exercise which stabilized the energy supplement that lowered the burden of oxidative phosphorylation to decrease lactate production. It also helped decrease the utilization of glycogen and protein which saved the protein consumption and lowered the production of BUN.25 Additionally, RHR protected muscles from mitophagy by suppressing the PINK1/Parkin signaling pathway under high oxygen stress and enhanced the activity of Na+-K+-ATPase. Sufficient ATP normalized the contraction of muscle and prevented muscle damage, which decreased the accumulation of CK during exercise.34 In summary, the anti-fatigue function of the RHR supplement might be due to the modulation of the energy metabolism to decrease the accumulation of fatigue metabolites. And its high anti-oxidant properties protect muscle from damage during exercise.
CONCLUSION
Supplementation with RHR supplements for four weeks enhanced exercise endurance in mice reduced the accumulation of fatigue substances such as lactate, CK, and BUN, and increased the storage of energy substance glycogen, which exhibits dose-dependent anti-fatigue effects. According to our findings, confirmed that RHR supplements were suitable for development as a dietary supplement with anti-fatigue properties, with an effective dosage of 600 mg/kg for mice, equivalent to a daily intake of 2926.4 mg of RHR supplements for humans.
INSTITUTIONAL REVIEW BOARD PERMISSION
Institutional Animal Care and Use Committee (IACUC) of National Taiwan Sport University (IACUC-11013).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








