INTRODUCTION
Osteoporosis is a major issue of epidemiology. According to the World Health Organization (WHO), osteoporosis is the second most serious epidemic in the world in chronic. The population above 50-years or with a high-risk of osteoporotic fracture in the world is estimated to be 158 million and is expected to double by 2040.1 Osteoporosis is a chronic and systemic disease of the bones. It progresses silently, has few apparent symptoms in the early stages, and most patients do not realize they have osteoporosis until they have a fracture.
The bones of the human body skeleton reach the peak at the age of 20 to 30-years. Bone mineral density decreases gradually with age, especially after the post-menopausal of females. Osteoclast activity increases due to estrogen deficiency, causing bone loss to accelerate. According to the International Osteoporosis Foundation (IOF), about 200 million women suffer from osteoporosis worldwide, and one of every third may experience a fracture due to osteoporosis after the post-menopausal. In order to reduce the occurrence of osteoporosis, promote health, and provide a good quality of life for seniors, the development of mild and long-term dietary supplements using natural constituents to help reduce bone loss and improve osteoporosis deserves more research.
Chicken sternal cartilage hydrolysate contains abundant collagen II, mucopolysaccharide, chondroitin, and hyaluronic acid, which are indispensable elements in bones and cartilage tissues. Some studies have indicated that the supplement of chicken sternal cartilage hydrolysate could improve the abnormal metabolism of cartilage and inhibit collagen degradation to reduce damage to the collagen structure. It has been shown to be effective in alleviating cartilage injury and has the potential to improve osteoarthritis.2
Soybean (Glycine max merrill) extract has a high isoflavone content. Isoflavone is a plant estrogen. One study indicated that after four weeks, oral administration of soybean isoflavone to women with the bones of the human body skeleton reach the peak at the age of 20 to 30. Bone mineral density decreases gradually with age, especially after the post-menopausal of females. Osteoclast activity increases due to estrogen deficiency, causing bone loss to accelerate. According to the IOF, about 200 million women suffer from osteoporosis worldwide, and one of every third may experience a fracture due to osteoporosis after the post-menopausal. The bone decomposition index of deoxypyridinoline content could be reduced significantly, osteoporosis could improve, and bones could be protected.3
Turmeric (Curcuma longa L.) extract has a high curcumin content, and it is anti-inflammatory and an antioxidant. Curcumin can alleviate knee pain and improve the life quality of patients with arthritis.4 Mangosteen (Garcinia mangostana L.) extract has a high α-mangostin content and antioxidant and anti- inflammatory bioactivities. Mangosteen extract can reduce the inflammatory factors in arthritic rats, as well as achieve an anti-inflammatory effect and alleviate pain effectively.5
Calcium is the principal component of the human body skeleton. The calcium absorption ability of the body declines with age, and when food intake is insufficient, calcium deficiency, bone loss, and osteoporotic fractures are likely to occur. Therefore, supplementing calcium appropriately is beneficial to bone health. Together with the synergetic effect of vitamin D and vitamin C, calcium absorption can be promoted and enhanced, bones can be strengthened, and bone health can be promoted.6
The benefits of the above-mentioned components to the human body are known, but no novel combination for improving osteoporosis has not yet been studied or discussed in practice. This study discussed the use of the chicken sternal compound (CSC) and used the osteoporosis-inducing model of bilateral ovariotomy in female Sprague-Dawley (SD) rats to evaluate the effect of the supplement developed in this study on bone health.
MATERIALS AND METHODS
Test Substances
The CSC was provided by HealthTake Corporation (820 mg per tablet). The formula contained chicken sternal cartilage hydrolysate (30.5%), turmeric extract (5.5%), mangosteen extract (12.2%), soybean extract (3%), calcium citrate (24.4%), salmon nasal cartilage extract, vitamin C, and vitamin D, and each tablet contained 10 mg of isoflavone.
Experimental Dose
The recommended dosage of CSC for adult humans is four tablets per day (820 mg per tablet). The oral dose for rats was calculated according to the metabolic conversion ratio of 6.2 between humans and rats. The rats were dosed with 339, 678 and 1356 mg/kg of CSC per day, which were one, two, and four times of the human dose, respectively, and 33.9, 67.8 and 135.6 mg/mL of CSC suspensions were prepared using 0.5% carboxymethylcellulose (CMC). The rats were dosed at 1 mL/100 g of body weight. The rats in the sham group and the ovariectomy (OVX+CMC) group were given CMC solutions with the same volume. The positive control (AD) group was dosed with alendronate at 2.5 mg/ kg7 and was dosed via oral administration three times a week (a 0.25 mg/mL suspension prepared with CMC).
Study Design
The animal experiment was approved by the China Medical University Institutional Animal Care and Use Committee (No: CMUIACUC-2020-282-1) and was performed according to the codes of ethics for institutional animals of China Medical University. A total of 72 eight-week-old female SD rats were purchased from BioLASCO Taiwan and reared in the animal center of China Medical University. The rearing condition was controlled in an environment alternating between light and dark at a constant temperature of 22±4 °C and constant humidity for 12-hours. Ovariectomies were performed at week 10 on the ovaries on both sides of the back of each rat. The rats were narcotized with pentobarbital sodium (40 mg/kg, i.p.). In addition, the sham group was incised, but not ovariectomized. After each rat was sacrificed, the ovarian tissues were checked to verify whether the ovariectomy was successful, and the experimental results of the failed animals were not adopted. The rats were divided into the sham group (10 rats) and five ovariectomy (OVX) groups (53 rats). The five groups of ovariectomized rats included one solvent group (OVX+CMC, 11 rats), one positive control group consisting of OVX+alendronate (OVX+AD, 10 rats), and three test material groups (OVX+CSC-L, 339 mg/kg; OVX+CSC-M, 678 mg/kg; and OVX +CSC-H, 1356 mg/kg respectively).
The various groups were dosed with the test materials two weeks after the ovariectomy operation and weighed once per week as a reference of the CSC dose for the particular rat of the week. The experiment lasted 14-weeks. After 14-weeks’ of administration of the test materials, the rats were narcotized, blood was drawn from the abdominal aorta, and two femora were taken out for analysis. The experiment design was shown in Figure 1.
Figure 1. Schematic Representation of the Experimental Design Timeline
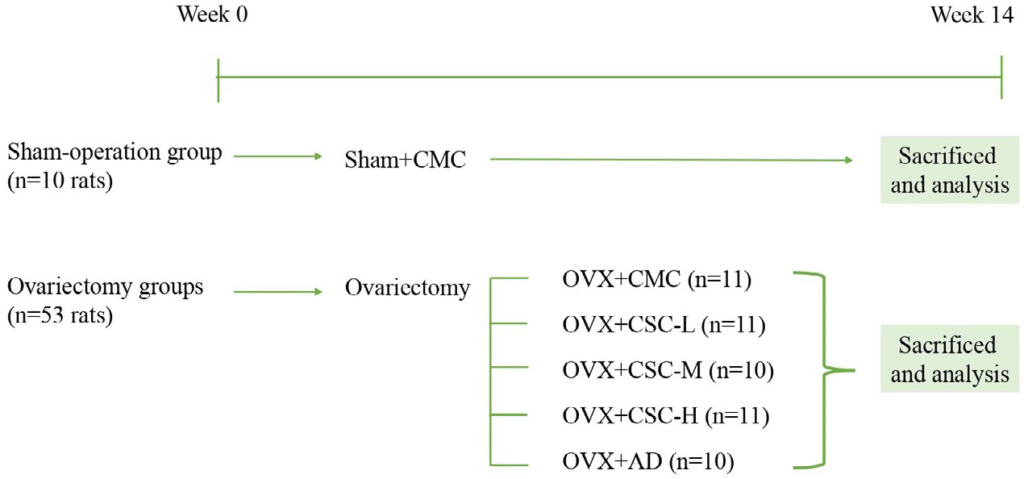
Sham+CMC, Sham-Operation Group, 0.5% CMC in diet (n=10); OVX+CMC, 0.5% CMC in diet after ovariectomy (n=11); OVX+CSC-L, 339 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (n=10); OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+AD, 2.5 mg/kg/3 times a week of AD in diet after ovariectomy (n=10).
Body Height, Weight and Food Intake
The body length and body length plus a tail length of the rats were measured weekly during the experiment, and the rats were weighed at the same time. Two to three rats were reared in each rearing box, the feed was weighed, and the daily average food intake per rat was estimated and recorded.
N-terminal Telopeptides of the Type I Collagen and Osteocalcin Concentrations in the Serum
The N-terminal telopeptides of the type I collagen (NTX) in the rats were determined by using the commercially available ELISA kit for cross linked N-telopeptide of type I collagen (Rat) reagent (Cloud- Clone Corp, TX, USA) on the principle of enzyme immunoassay, according to the operation instruction. The procedure is briefly described below. An amount consisting of 50 μL of the standard sample and the serum sample was put in the 96-well plate of the kit, and 50 μL of detection reagent A was put in each well. The well plate reacted at 37 °C for one hour, and then the well plate was washed with a buffer solution. An amount consisting of 100 μL of detection reagent B was then put in the well plate, and the well plate reacted at 37 °C for 30 minutes. The well plate was washed with a buffer solution, filled with 90 μL of a substrate solution, and then reacted at 37 °C for 20-minutes. Finally, each well was filled with 50 μL of a stop solution to stop the reaction. The absorbance value of the well plate at 450 nm was tested by a spectrophotometer, the standard regression curve of the absorbance value corresponding to the standard concentration was made, and the curvilinear equation was obtained. The sample absorbance value was substituted into the curvilinear equation, the dilution factor was restored, and the NTX concentration in the serum of each rat was calculated.
The rats’ osteocalcin (OCN) levels were determined using the commercially available Rat Osteocalcin ELISA Kit (Cat. No. BT-490, Biomedical Technologies Inc., MA, USA) on the principle of enzyme immunoassay, according to the operation instruction. The procedure is briefly described below. An amount consisting of 100 μL of the standard samples at different concentrations (0.25-20 ng/mL) and the rat serum diluent (five time dilution using a sample buffer solution) was put in the appended 96-well plate of the kit and reacted at 2-8 °C for 24-hours. The well plate was washed with a phosphate buffer, after which 100 μL of osteocalcin rat antiserum was put in the well plate and reacted at 37 °C for one hour. Afterwards, the well plate was washed with the phosphate buffer according to the cleaning step. Each well was then filled with 100 μL of donkey anti-goat immunoglobulin G (IgG) peroxidase and reacted at room temperature for one hour. Then, each well is filled with 100 μL of a tetramethylbenzidine/hydrogen peroxide (TMB/H2 O2 ) mixed solution and reacted at room temperature in the dark for 30-minutes. Finally, 100 μL of a stop solution was applied to stop the reaction. The absorbance value of the well plate at 450 nm was tested with a spectrophotometer, the standard regression curve of the absorbance value corresponding to the standard concentration was made, and the curvilinear equation was obtained. The sample absorbance value was substituted into the curvilinear equation, the dilution factor was restored, and the OCN concentration in the serum of each rat was calculated.
Bone Tissue Analysis
The right femur was taken out after each rat was sacrificed, and a computed tomography (CT) image of the femur was captured using micro-computed tomography at a resolution of 18 μm. The trabecular area percentage, bone and tissue volume, trabecular thickness, trabecular number, and trabecular separation were analyzed by analytical software. The analytic position of distal diaphysis on the right femur was the region from the growth plate to 2,700 μm below the growth plate (towards the proximal end), excluding the cortical bone. The femoral bone mineral density was analyzed using the same region but included the cortical bone.
Biology Analysis
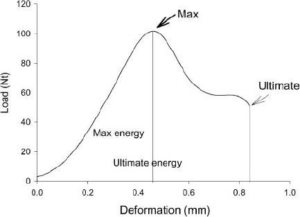
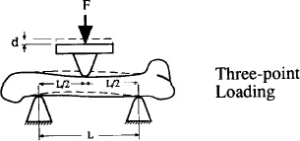
The three-point bending was determined by a bone stress determinator (RT1-TST, Royalty Tec. Ins. LTD, Taiwan). The fixed points at both ends were 5 mm, respectively, and the speed was 0.5 mm/min. The force and distance were recorded, and the crosssectional area was worked out by image analysis software after tomography. The femur was analyzed by the test system, and the obtained parameters of the bone mechanics analysis included the maximum load (i.e., the force converted from the recorded maximum weight), with Newton (N) as the unit. The absorbed energy was the integral of the area under curve, and the unit was mJ (i.e., N multiplied by distance), as shown in Figure A. The stiffness was represented by the slope, and the N of the maximum load was divided by the distance (N/mm). Young’s modulus, or the elastic modulus (E), was calculated using the following equation8,9:
pressure σ= FLc/4I
torsion E=F/d×L3 /48I
E is elastic modulus
F is the applied load (N)
c is the distance from the center of mass (equal to ½b)
d is the displacement (mm)
L is the span between the two support points of the bending fixture (mm) (as shown in Figure B)
I is the cross-sectional moment of inertia
I=π[ab3 -(a-2t) (b-2t)3 ]/64
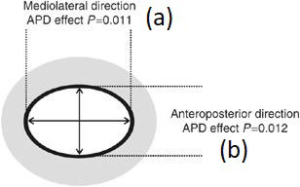
a: mediolateral direction (as shown in Figure C, D)
b: anteroposterior direction (as shown in Figure C, D)
t: the average of the cortical thickness

Statistical Analysis
The data derived from this test were analyzed by one-way analysis of variance and Duncan’s multiple range test was performed. The statistical result was represented using English letters, with the same letter indicating no statistical difference between groups.
RESULTS
Influence on Body Weight
As shown in Table 1, two weeks after the ovariectomy, the rat body weight of the OVX+CMC group was significantly higher than that of the sham group, and the average weight of the groups of three doses of OVX+CSC and the OVX+AD group was not different from that of OVX+CMC group. From week 3 to week 16 after ovariectomy, the rat body weight of the OVX+CMC group remained significantly higher than that of the sham group; from week 3 to week 16 after ovariectomy, the rat body weight of the groups of three doses of OVX+CSC and the OVX+AD group was not different from that of the OVX+CMC group.
| Table 1. Effect of the Supplement on the Body Weight of the Ovariectomized Rats |
|
Body Weight (g)
|
|
Weeks#
|
Sham+CMC |
OVX+CMC |
OVX+CSC-L |
OVX+CSC-M |
OVX+CSC-H |
OVX+AD
|
|
2
|
254.4±12.8a |
286.3±23.3b |
286.1±15.2b |
285.2±13.9b |
285.9±15.7b |
286.5±12.8b |
|
3
|
263.5±13.3a
|
310.8±23.4b |
307.5±15.2b |
308.9±22.0b |
305.1±19.2b |
311.8±14.6b
|
| 4 |
273.6±15.4a |
331.3±26.3b |
326.1±16.8b |
326.7±22.0b |
322.1±18.0b |
329.6±15.8b
|
|
5
|
276.8±15.0a |
338.4±26.2b |
333.9±18.2b |
334.4±23.0b |
331.0±19.4b |
339.5±15.3b
|
|
6
|
286.3±18.1a |
354.4±32.3b |
344.8±20.1b |
348.1±27.7b |
343.5±20.8b |
349.8±21.1b
|
|
7
|
289.3±18.2a |
361.5±32.1b |
352.8±20.5b |
355.3±27.2b |
351.2±22.3b |
357.4±20.6b
|
|
8
|
293.6±18.6a |
366.7±32.3b |
359.4±21.9b |
363.2±27.7b |
358.5±23.5b |
367.9±21.3b
|
|
9
|
296.8±19.7a |
378.0±34.4b |
369.6±22.4b |
372.2±27.7b |
366.5±24.8b |
376.1±24.3b
|
|
10
|
298.6±20.6a |
382.6±35.2b |
374.2±24.7b |
375.6±29.7b |
371.8±24.0b |
378.7±24.6b
|
|
11
|
300.4±21.5a |
386.5±34.2b |
378.7±24.8b |
378.9±29.6b |
375.9±25.5b |
381.9±24.7b |
|
12
|
303.3±20.9a |
391.8±34.3b |
383.3±25.9b |
383.2±29.8b |
379.1±26.5b |
387.1±26.6b
|
| 13 |
305.0±20.8a |
394.6±34.3b |
387.3±27.1b |
386.3±29.8b |
380.9±27.1b |
390.4±26.6b
|
|
14
|
308.3±22.1a |
400.1±35.4b |
393.9±27.9b |
392.8±29.5b |
387.0±27.7b |
395.4±27.2b
|
|
15
|
310.7±20.7a |
406.9±37.5b |
400.0±28.0b |
400.6±28.9b |
393.5±31.0b |
402.6±28.0b
|
|
16
|
314.1±20.9a |
410.6±37.6b |
405.3±28.5b |
403.1±31.3b |
397.4±30.2b |
407.3±29.1b
|
| Sham+CMC, Sham-Operation Group, 0.5% CMC in diet (n=10); OVX+CMC, 0.5% CMC in diet after ovariectomy (n=11); OVX+CSC-L, 339 mg/kg/day of csc in diet after ovariectomy (n=11); OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (n=10); OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+AD, 2.5 mg/kg/3 times a week of AD in diet after ovariectomy (n=10). Values were expressed as Means±SD. Data were analyzed by one-way analysis of variance using the Duncan’s multiple range test. The statistical results are represented by letters, and the same letters represent no statistical difference between groups (p>0.05). #weeks after ovariectomy in rats. |
Influence on NTX and OCN Concentrations in Serum
As shown in Table 2, the NTX and OCN concentrations in the rat serum of the OVX+CMC group were significantly higher than that of the sham group. In addition, the NTX and OCN concentrations in the serum of the groups of three doses of OVX+CSC and the OVX+AD group were significantly lower than that of the OVX+CMC group.
| Table 2. Effect of Supplement on the NTX and OCN Concentrations in the Serum of the Ovariectomized Rats |
|
Treatments
|
NTX (ng/mL) |
OCN (ng/mL)
|
|
Sham+CMC
|
10.0±0.9a |
16.4±3.2a |
| OVX+CMC |
13.4±1.4d |
28.8±2.0d
|
|
OVX+CSC-L
|
12.5±1.8c |
24.3±3.6c |
| OVX+CSC-M |
11.8±2.0c |
24.3±4.2c
|
|
OVX+CSC-H
|
11.7±1.9bc |
21.4±3.8bc |
| OVX+AD |
11.4±1.1b |
0.3±3.2b
|
| Sham+CMC: Sham-Operation Group, 0.5% CMC in diet (n=10); OVX+CMC, 0.5% CMC in diet after ovariectomy (n=11); OVX+CSC-L, 339 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (n=10); OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+AD, 2.5 mg/ kg/3 times a week of AD in diet after ovariectomy (n=10). Ntx, N-Terminal Telopeptides of type I Collagen; OCN: Osteocalcin. Values were expressed as Means±SD. Data were analyzed by one-way analysis of variance using the Duncan’s multiple range test. The statistical results are represented by letters, and the same letters represent no statistical difference between groups (p>0.05). |
Influence on Right Femur Length and Bone Mineral Density
As shown in Table 3, the right femur length of the rats in the OVX+CMC group was significantly longer than that of the sham group, and the rats’ right femur length of the groups of three doses of OVX+CSC was not different from that of the OVX+AD group.
| Table 3. Effect of Supplement on the Femur Length and Bone Mineral Density of the Ovariectomized Rats |
|
Treatments
|
Femur Length (mm) |
Bone Mineral Density (g/cm3 )
|
|
Sham+CMC
|
34.6±0.4a |
0.66±0.06c
|
|
OVX+CMC
|
35.3±0.6b |
0.53±0.02a |
|
OVX+CSC-L
|
35.3±0.6b |
0.57±0.04b |
|
OVX+CSC-M
|
35.4±0.7b |
0.57±0.05b
|
| OVX+CSC-H |
35.2±1.1ab |
0.59±0.04b
|
| OVX+AD |
35.3±0.3b |
0.65±0.04c
|
| Sham+CMC, Sham-Operation Group, 0.5% CMC in diet (n=10); OVX+CMC, 0.5% CMC in diet after ovariectomy (n=11); OVX+CSC-L, 339 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (n=10); OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+AD, 2.5 mg/kg/3 times a week of AD in diet after ovariectomy (n=10). Values were expressed as Means±SD. Data were analyzed by one-way analysis of variance using the Duncan’s multiple range test. The statistical results are represented by letters, and the same letters represent no statistical difference between groups (p>0.05). |
The bone mineral density of the right femur was analyzed by micro-computed tomography. As shown in Table 3, the bone mineral density of the OVX+CMC group was significantly lower than that of the sham group. The bone mineral density of the right femur of the groups of three doses of OVX+CSC and the OVX+AD group was significantly higher than that of the OVX+CMC group.
Influence on trabecular area percentage, thickness, numA B ber, and separation of the right femur. The right femur morphology was analyzed by micro-computed tomography. As shown in Figure 2 and Table 4, the trabecular area percentage, thickness, and number of the OVX+CMC group were significantly lower than that of the sham group. The trabecular area percentage and trabecular thickness of the groups of three doses of OVX+CSC and the OVX+AD group were significantly larger than that of the OVX+CMC group. The OVX+CSC-H group and OVX+AD group could significantly increase the trabecular number in comparison to the OVX+CMC group. The trabecular separation analysis showed that the trabecular separation of the OVX+CMC group was significantly higher than that of the sham group. The groups of three doses of OVX+CSC and the OVX+AD group could significantly reduce the trabecular separation in comparison to the OVX+CMC group.
Figure 2. Micro-Computed Tomography Analysis of the Femora
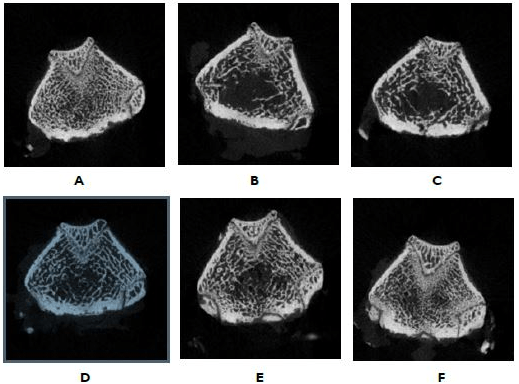
(A) Sham+CMC, Sham-Operation Group, 0.5% CMC in diet (BV/TV:49.6); (B) OVX+CMC, 0.5% CMC in diet after ovariectomy (BV/TV: 34.9); (C) OVX+CSC-L, 339 mg/kg/day of CSC in diet after ovariectomy (BV/TV: 38.5); (D) OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (BV/TV: 39.4); (E) OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (BV/TV: 41.2); (F) OVX+AD, 2.5 mg/kg/3 times a week of AD in diet after ovariectomy (BV/TV: 47.6). The image is at the growth plate. BV/TV: bone volume/ tissue volume.
| Table 4. Effect of Supplement on the Trabecular Area Percentage, Thickness, Number and Separation of Femora of the Ovariectomized Rats |
|
Treatments
|
BoneVolume/ Tissue Volume (%) |
Trabecular Thickness (μm) |
Trabecular Number (No./ mm) |
Trabecular Separation (μm)
|
|
Sham+CMC
|
48.1±5.5c |
176.9±12.8c |
3.7±0.2d |
150.9±18.9a |
|
OVX+CMC
|
35.2±1.5a |
150.9±6.8a |
2.7±0.2a |
302.2±42.2d
|
| OVX+CSC-L |
38.3±2.5b |
160.5±11.2b |
2.8±0.2ab |
261.5±30.7c
|
|
OVX+CSC-M
|
39.4±3.2b |
161.1±7.3b |
2.9±0.3ab |
241.8±45.9bc
|
|
OVX+CSC-H
|
41.2±2.8b |
162.0±9.2b |
2.9±0.1b |
217.5±18.0b
|
|
OVX+AD
|
47.3±4.4c |
167.0±7.9b |
3.1±0.2c |
69.4±16.5a
|
| Sham+CMC, Sham-Operation Group, 0.5% CMC in diet (n=10); OVX+CMC, 0.5% CMC in diet after ovariectomy (n=11); OVX+CSC-L, 339 mg/kg/day of csc in diet after ovariectomy (n=11); OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (n=10); OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+AD, 2.5 mg/kg/3 times a week of AD in diet after ovariectomy (n=10). Values were expressed as Means±SD. Data were analyzed by one-way analysis of variance using the Duncan’s multiple range test. The statistical results are represented byletters, and the same letters represent no statistical difference between groups (p>0.05). |
Influence on maximum load, stiffness, absorbed energy, and Young’s modulus of the left femur. As shown in Table 5, the maximum load, absorbed energy, and Young’s modulus of the left femur of the rats in the OVX+CMC group was significantly lower than those of the sham group. In comparison to the OVX+CMC group, the groups of three doses of OVX+CSC and the OVX+AD group showed significant increases in the maximum load, stiffness, absorbed energy, and Young’s modulus of the left femur. The left femur morphology, as analyzed by CT, is shown in Figure 2, which indicates the trabecular area percentage, thickness, and a number of the OVX+CMC group are significantly lower than those of the sham group.
| Table 5. Effect of Supplement on the Maximum Load, Stiffness, Absorbed Energy, and Young’s Modulus of the Femora of the Ovariectomized Rats |
|
Treatments
|
Max Load (N) |
Stiffness (N/mm) |
Energy (mJ) |
Young’s Modulus (GPa)
|
|
Sham+CMC
|
149.2±19.2c |
137.4±26.5b |
113.1±18.8b |
5.1±1.3d |
|
OVX+CMC
|
107.2±13.2a |
105.8±20.9a |
79.9±10.1a |
3.0±0.7a |
| OVX+CSC-L |
128.4±10.4b |
128.3±26.1b |
100.0±18.1b |
3.8±1.1b
|
| OVX+CSC-M |
35.8±10.4bc |
130.2±11.7b |
105.4±14.3b |
4.1±0.8bc
|
|
OVX+CSC-H
|
136.6±18.4bc |
133.3±10.1b |
103.0±12.0b |
4.2±0.6bc
|
|
OVX+AD
|
44.4±15.4c |
141.5±17.0b |
109.7±17.0b |
4.8±0.9cd
|
| Sham+CMC, Sham-Operation Group, 0.5% CMC in diet (n=10); OVX+CMC, 0.5% CMC in diet after ovariectomy (n=11); OVX+CSC-L, 339 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+CSC-M, 678 mg/kg/day of CSC in diet after ovariectomy (n=10); OVX+CSC-H, 1356 mg/kg/day of CSC in diet after ovariectomy (n=11); OVX+AD, 2.5 mg/kg/3 times a week of AD in diet after ovariectomy (n=10). Values were expressed as Means±SD. Data were analyzed by one-way analysis of variance using the Duncan’s multiple range test. The statistical results are represented by letters, and the same letters represent no statistical difference between groups (p>0.05). |
DISCUSSION
Osteoporosis is likely to occur in post-menopausal women. Osteoclast activity increases due to estrogen deficiency and promotes the bone erosion effect.10 Estrogen becomes deficient after menopause, osteoporosis is accelerated, the bone density decreases by 2-5% annually, and the duration of this bone loss lasts for ten years.11 Therefore, this test used the ovariectomized rat model to evaluate the effect of CSC on resisting bone loss. Prior toxicity test results have shown that CSC supplements are safe.12
Food intake and body weight will increase as a result of estrogen deficiency.13,14 The food intake of the rats in the OVX groups increased in the first six weeks of the test, and the body weight was obviously larger than that of the sham group in the process, meaning the post-menopausal symptoms in the experimental animals were successfully presented by the ovariectomy procedure.
The determination of biochemical indexes is unharmful. The test reagents for evaluating osteoporosis have been invented clinically, which can detect osteoblast or osteoclast activity. OCN is a common biochemical index of bone remodeling effect for bone formation, and NTX is a common biochemical index of bone remodeling effect for bone resorption. OCN is a specific non-collagen protein secreted from osteoblasts; most OCN is combined with hydroxyapatite to form the bone matrix, and a small part of it gets into the blood. OCN is released during bone resorption; therefore, it is a sensitive index of bone formation. OCN increases with bone turnover rate.15 The stability of collagen depends on the cross-link of pyridinoline (Pyd) or deoxypyridinoline (Dpd) at two terminals. When collagen is damaged by the bone resorption effect, Pyd or Dpd is released, and the concentrations of these two substances can be detected to evaluate the bone resorption effect. Type I collagen has aminoterminal (N-terminal) and carboxy-terminal (C-terminal) peptide- attached cross-links, and the antibodies against NTX and Cterminal telopeptide (CTX) have already been developed, which can be determined by immunoreaction.15
In this test, the NTX concentration in the blood increased 16-weeks after the rats were ovariectomized; i.e., the osteoclast activity increased, the bone turnover rate increased, and the OCN concentration in the blood increased. CSC can reduce the NTX and OCN concentrations in the blood, and CSC may reduce bone loss by inhibiting the activity of osteoclasts.
In recent years, due to molecular imaging medicine advances, computed tomography has been applied to small animals. Micro-computed tomography is the optimal instrument for studying bone structures, as the stereo scanning data of bone structures can be used for analyzing bone mineral density, the trabecular number and thickness, the trabecular separation, and the trabecular area percentage,16 making it suitable as a replacement for bone mineral density analysis using a dual-energy Xray absorptiometer and traditional pathological section analysis. The right femur was analyzed by micro-computed tomography 16-weeks after the rats were ovariectomized, and the OVX+CSCL group showed improved bone mineral density, trabecular area percentage, trabecular thickness, and trabecular separation, meaning the use of CSC improved the bone tissue morphology.
Osteoporosis is likely to cause fractures. The analysis of bone tissue and the measurement of bone mineral density are insufficient to fully show the deterioration of bones.17 The use of diphosphate drugs to treat osteoporosis can improve bone mineral density, but fracture risks still remain after long-term usage.18 Therefore, besides the measurement of bone mineral density, the determination of bone biomechanics was necessary. This study used three-point bending to assay the maximum load, stiffness, absorbed energy, and Young’s modulus of the femora, and the result show that the use of CSC could improve the maximum load, stiffness, absorbed energy, and Young’s modulus of the femora, which have been induced by the ovariectomy of rats.
The isoflavone in soybeans is mainly in the glucoside and aglycone forms. The isoflavone in the glucosides has no bioavailability, meaning it must be digested and hydrolyzed before it is absorbed, whereas the isoflavone in the aglycones can be absorbed directly and used quickly. When high-dose glucoside isoflavone or aglycone isoflavone was administered in one single time, the result show that the isoflavone concentration in the plasma of the subjects receiving aglycone isoflavone was more than five times that of the subjects receiving glucoside isoflavone. This result indicated that the aglycone isoflavone had better absorption utilization. The soybean extract in the CSC was 40% aglycone isoflavone, which can be absorbed by the human body faster.19 Multiple prior studies have indicated that soybean isoflavone is helpful for preventing the loss of bone mineral density.20-22
Eating turmeric, which has a high curcuminoid content, can inhibit the trabecular loss induced by OVA and maintain the quantity and supporting force of strut-like trabeculae. The findings showed that turmeric could protect bones.23 Another experiment indicated that turmeric can reduce the formation of osteoclasts and impaire receptor activator of nuclear factor-kappa (RANK) signaling, making it beneficial for protecting against osteoporosis induced by estrogen deficiency.24 Some studies have proved that it can prevent the differentiation of osteoclasts and maintain bone mineral density.23,25 The use of turmeric extract in the CSC is helpful for bone protection.
Studies have shown that when the mice with bone destruction were dosed with mangosteen extract, mangosteen extract could inhibit the formation of osteoclasts and maintain bone health.26,27 Another experiment showed that mangosteen extract can inhibit the interleukin 1β in rats with osteoarthritis, as well as prevent cartilage degradation.28
Chicken sternal cartilage hydrolysate has a high content of collagen II, which is the main component in cartilage as proven by many pieces of literature and can prevent osteoporosis and osteoarthritis.29-31
LIMITATION
The preliminary significant results can be found in the present animal experiment. Nonetheless, whether the effect of long-term administration on humans is correlated with bone health should be observed in the future. This is a topic worthy of in-depth discussions.
CONCLUSION
The animal experiment results of this study show that for the osteoporosis of the ovariectomized rats, the dose of 1 time concentration of CSC could significantly increase the bone mineral density, trabecular area percentage, and trabecular thickness, as well as reduce the trabecular separation significantly. The index of serum collagen loss, NTX, and the concentration OCN which is an index of bone turnover rate could effectively retard the bone loss induced by ovariectomy and achieve the effect of bone health. Hence, CSC has the potential to be a new bone health supplement and should be considered for testing on the human body in the future. The lasting effect on the human body requires further research, so that possible variables can be improved.
INSTITUTIONAL REVIEW BOARD APPROVAL
The animal experiment was approved by the China Medical University Institutional Animal Care and Use Committee (No: CMUIACUC-2020-282-1) and was performed according to the codes of ethics for institutional animals of China Medical University. A total of 72 eight-week-old female Sprague-Dawley rats were purchased from BioLASCO Taiwan and reared in the animal center of China Medical University.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.











