INTRODUCTION
Synthetic pyrethroids have been widely used for more than three decades to manage pests in a variety of crops,1 but they have become increasingly popular following a complete ban on the use of cholinesterase-inhibiting insecticides.2,3 The pyrethroids constitute a high share of the insecticide market because their activity profiles indicate high efficiency, low mammalian and avian toxicity, and biodegradability.4 Today, pyrethroids are used in agriculture, forestry, horticulture, public health, and are active ingredients of many insect-controlling products intended for indoor use.3 Based on the symptoms generated in animals, pyrethroids fall under two distinct classes: Types I and II. Type I pyrethroids affect the sodium channels in nerve membranes and type II pyrethroids cause an even longer delay in the sodium channel inactivation leading to persistent depolarization of the nerve membrane without monotonous discharge. The pyrethroids are more hydrophobic in nature,5 and their target site of action is the biological membrane. In addition, type II pyrethroids affect the central nervous system (CNS), while type I affects the peripheral nerves.6 To analyze the effects of suspected toxic substances, hemato-biochemical studies are essential. Recent studies have reported that pyrethroids lead to significant alterations as was observed on the basis of hematological findings.7 Moreover, the liver, being the primary site for pyrethroid metabolism ultimately led to liver damage, and was found to accumulate large amounts of pyrethroid residues.8 Several investigations have reported that pesticides adversely affect testicular functions in experimental animals6,9,10,11 and function as potent endocrine disrupters.12,13 Few reports have demonstrated the induction of oxidative stress by pyrethroids.12,13,14,15 CYP is an insecticide that belongs to the family of synthetic pyrethroids. Its chemical formula is alpha-cyano-3-phenoxybenzyl ester of 2,2 dimethyl-3- (2,2 dichlorovinyl)-cyclopropane carboxilic acid,, the most widely used type II pyrethroid pesticides. It is commonly used to control moths and pests of cotton, soyabean and other crops. It was believed that CYP showed low mammalian toxicity so it was also used for controlling household pests. However, a number of studies have proven that CYP and other pyrethroids have hepatotoxic, carcinogenic, neurotoxic, and immunosuppressive potential in mammals.16,17,18 Synthetic pyrethroids also have the ability to disrupt biochemical, hematological, and reproductive parameters.19 The toxicity of pyrethroid insecticides in mammals have received great attention in the recent years because animals exposed to these insecticides have exhibited changes in their physiological activities besides other pathological features.20 Oxidative stress and reactive oxygen species so produced in response to stress, as well as endocrine disturbance are among the most significant effects of pesticide toxicology.21,22,23,24,25 CYP may also induce oxidative stress and endocrine disturbance in organisms. Giray et al14 also reported that oral administration of CYP to rats induce significant oxidative stress in cerebral and hepatic tissues. Recently, it was proven that exposure to CYP during lactation significantly decreased the layer of spermatogenic cells, increased the inside diameter of seminiferous tubules, and disturbed the array of spermatogenic cells in the testes of pups on postnatal day 21.26
For any type of pesticide, the exposure concentration is important in analyzing the variation of its toxicity. So, in this piece of work, Wistar rats were exposed to different concentrations of CYP to study the impact of CYP on hematological, hepatic and gonadal parameters in rats.
MATERIALS AND METHODS
Chemicals and Reagents
A commercial formulation of cypermethrin [(RS) -α -cyano-3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl) 2,2-dimethylcyclopropanecarboxylate] 10% emulsifiable concentrate (EC), named ‘‘Ustad’’ (United Phosphorus Limited) was used in the experiments. H2O2, red blood cell dilution fluid, white blood cell dilution fluid, Drabkin’s diluents, aspartic acid, α-ketoglutaric acid, NaOH, DL-alanine,2,4-dinitrophenylhydrazine hydrochloride (DNPH), sodium dodecyl sulfate (SDS), thiobarbituric acid, n-Butanol-pyridine, acetate buffer, sulfosalicylic acid, dithionitrobenzoic acid (DTNB), HCl, Tris-HCl, para-nitrophenol phosphate, ZnSO4, ferric chloride, glacial acetic acid, cholesterol, NaCl, phosphate buffer, Na2HPO4, KH2PO4, NaH2PO4, NaOH, DL-alanine, α-Ketoglutaric acid, glycerol, ethylenediaminetetraacetic acid, pentobarbital sodium, TNaPP, nicotinamide adenine dinucleotide, testosterone, dehydroepiandrosterone, and other chemicals were procured from Sigma-Aldrich, St. Louis, MO, USA; Merck India, Ltd., Mumbai, India and Himedia India, Ltd., Mumbai, India.
Animal Care and Maintenance
For the present study, mature Wistar male and female albino rats (weighing 130-150 g) were taken and the animals were accommodated in labeled cages with solid plastic sides and stainless-steel grid tops and floors, in a temperature controlled room (approximately 25±2 °C), and light cycle (12 h light, 12 h dark). The rats were acclimatized for one week prior to the different modes of treatment. Animals were fed a standard laboratory pellet diet and water ad libitum. This study was permitted by the Institutional Animal Ethical Committee (IAEC), registered under the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Experimental Design
This study was undertaken in accordance to the OECD-423 guidelines. Mature male and female rats were divided into nine groups where each group contained six animals. Group I was designated as the control and Groups II to IX were CYP-treated, were administered with 1/11, 1/10, 1/9, 1/7, 1/5, 1/4.5, 1/4 and 1/3 LD50 of CYP-EC respectively. According to the Food and Agriculture Organization (FAO) report,27 an oral LD50 dose of CYP for male and female rats were of the body weight 360 and 309 mg/kg respectively.28 Following the oral treatment of CYP for 14 consecutive days, all animals were anesthetized with pentobarbital sodium on the 15th day and sacrificed by cervical dislocation. Tissue samples (liver, testis and ovary) were collected and stored at -80 0C until analysis. For sperm collection, epididymis was collected and washed immediately.
Blood Withdrawal Technique
Blood samples were drawn from hepatic vein of all animals using a syringe and collected in graduated centrifuge tubes containing anticoagulant ethylenediaminetetraacetic (EDTA) for the estimation of hematological parameters. For the estimation of serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT), serum was prepared from blood which was not treated with EDTA.
Study on Body Weight
During the treatment schedule, the body weight of each animal that was put under fasting overnight was measured regularly before each treatment. Following 24 h of the last dose, the weight of all the animals were recorded, following which they were sacrificed.
Study of Haematological Parameters in Male Rat
Blood cell count and Hb%: The total erythrocyte count was recorded after diluting the blood sample at 1:200 with the RBC dilution fluid. The total RBC counts29 were recorded using the Neubaur hemocytometer and expressed as x106 mm-3. Blood was diluted in a ratio of 1:20 with the WBC dilution fluid. Using Neubaur hemocytometer, the total count for leukocytes was recorded,29 and the counts were expressed as x103 mm-3.
Using the cyanomethemoglobin method,30 the hemoglobin percentage was measured. 20 μl of blood and 5 ml of Drabkin’s solution were added in a test tube and the optical density was measured at 540 nm.
Estimation of serum glutamate-oxalacetate transaminase (SGOT) and serum glutamate-pyruvate transaminase (SGPT): Briefly, to 1.0 ml of the buffered substrate (200 mM/L of DL-aspartate and 2 mM/L of α-ketoglutarate, pH=7.4), 0.1 ml of serum was added and incubated at 37 °C for 1 h. Then, 1 ml of dinitrophenyl hydrazine (DNPH) was added and kept for 20 minutes at room temperature. After 20 minutes, 10 ml of 0.4 N sodium hydroxide (NaOH) was added, and the color intensity was measured at 520 nm in a spectrophotometer (UV-Shimadzu-245, Japan) after 10 minutes against the blank. For the estimation of SGPT, to 1.0 ml of the buffered substrate (200 mM/L of DL-alanine and 2 mM/L of α- ketoglutarate pH=7.4), 0.1 ml of serum was added and incubated at 37 °C for 1 h. The reaction was arrested by the addition of 1.0 ml of DNPH and left aside for 20 min at room temperature. The color developed by the addition of 10 ml of 0.4N NaOH was read at 520 nm in spectrophotometer (UV-Shimadzu-245, Japan)31 against the blank. The enzyme activity in serum was expressed as IU/lit.
Estimation of Malondialdehyde (MDA)
Malondialdehyde estimation was also performed according to the modified method of Ohkawa et al.32 Briefly, 1 ml of the sample was mixed with 0.2 ml of 8.1% SDS, 1.5 ml of 20% acetate buffer (pH-3.5) and 1.5 ml of 0.8% aqueous solution of thiobarbituric acid and the mixtures were boiled for 60 min at 95 ˚C. The red pigment produced after heating was extracted with 5 ml of n-butanol-pyridine (15:1) mixture and centrifuged at 5000 rpm for 10 min at room temperature. The absorbance of the supernatants was recorded at 535 nm.
Assay of Reduced Glutathione
Reduced glutathione estimation was performed using the method of Griffith.33 First, 100 µl of sulfosalicylic acid was mixed with 200 μl of liver homogenate, and the mixture was centrifuged for 10 min at 3000 rpm. Then, 1.8 ml of DTNB was added to the supernatant and was shaken well. The reading was taken at 412 nm. The glutathione level in tissue was expressed as µg/mg protein.
Sperm Count
Sperm suspension from the caudal epididymis was diluted using a sperm dilution fluid and added to the counting chamber. The count of spermatozoa was recorded34 using a hemocytometer under the light microscope. The sperm concentration was expressed as x106 ml-1.
Determination of Testicular and Ovarian Cholesterol
Tissue homogenate (20 mg/ml) was prepared with 0.5% FeCl3 solution and was centrifuged at 2000 rpm to collect the supernatant. Next, to 0.1 ml of supernatant, 6 ml of glacial acetic acid was added to prepare the sample. Simultaneously, 5.9 ml of glacial acetic acid was also taken in a separate test tube with 0.1 ml of the working standard (0.2 mg/ml) and 0.1 ml of distilled water was used to prepare the standard. The blank was prepared by mixing 6 ml of glacial acetic acid and 0.1 ml of distilled water in another test tube. Then, 4 ml of colored reagents were added to each test tube and mixed vigorously, and was allowed to stand for 20 minutes for the spectrophotometric reading at 570 nm against the blank.35
Estimation of testicular ∆5, 3β-hydroxy steroid dehydrogenase (∆5, 3β-HSD): The estimation of testicular ∆5, 3β-hydroxy steroid dehydrogenase (∆5, 3β-HSD) was performed according to the protocol of Talalay.36 Testicular ∆5, 3β-HSD homogenizing media was prepared by adding 20 ml of glycerol, and 0.01 M EDTA in 0.05 M phosphate buffer to 100 ml of redistilled water. Following the centrifugation of testicular homogenate at 10,000 rpm for 30 min at 4 °C in a cold centrifuge, 1 ml of the supernatant was mixed with 0.9 ml of distilled water, 1 ml of sodium pyrophosphate buffer, and 40 µl of dehydroepiandrosterone (DHEA). The activity of ∆5,3β-HSD was measured following the addition of 0.1ml of NAD, at 340 nm against a blank (without NAD).Estimation of ovarian 17β HSD: Tissue homogenate (20 mg/ml) was centrifuged at 10,000 rpm for 30 min at 4 °C in a cold centrifuge and the supernatant was collected. The supernatant (1ml) was mixed with 1 ml of sodium pyrophosphate buffer and 40 µl of dehydroepiandrosterone (DHEA). For 17ß HSD activity measurements,37 the same supernatant fluid was added into 440 µM of sodium pyrophosphate buffer, 960 µl of bovine serum albumin, and 40 µl of ethanol containing testosterone. The enzyme activity was measured following the addition of NAD to the tissue supernatant mixture in a spectrophotometer at 340 nm against a blank (without NAD). Result is expressed as one unit of enzyme activity which is equivalent to a change in the absorbance of 0.001/min at 340 nm.
Statistical Analysis
The result was expressed as mean±SEM. Statistical analysis was performed using a one-way analysis of variance (ANOVA) using Origin 6.1 software. The difference was considered as statistically significant when p<0.05.
RESULTS
Alterations in Body Weight, Food and Water Intake
Significant alterations in the final body weight were observed at the dose level of 80 mg/kg body weight (1/4.5 LD50) in male rats (Figure 1) and at the dose level of 51.5 mg/kg body weight (1/6 LD50) in female rats (Figure 2).
Figure 1: Effect of Cypermethrin on Body Weight of Male Rat.
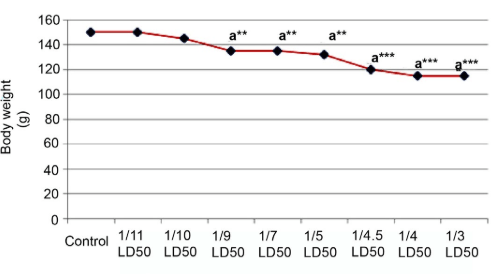 Results are Expressed as Mean±SEM. The Data has been Analyzed using one way ANOVA.
Results are Expressed as Mean±SEM. The Data has been Analyzed using one way ANOVA.
Superscript a, Control Group Versus All Other Groups (**indicates p<0.01 and ***indicates p<0.001).
Figure 2: Effect of Cypermethrin on Body Weight of Female Rat.
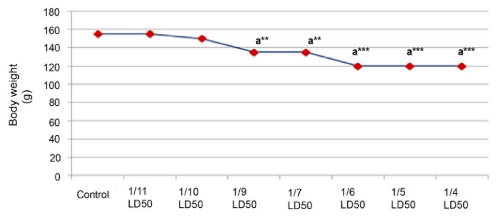 Results are Expressed as Mean±SEM. Data Analysis has been Performed Using One Way ANOVA.
Results are Expressed as Mean±SEM. Data Analysis has been Performed Using One Way ANOVA.
Superscript a, Control Group Versus Aall Other Groups (**Indicates p<0.01 and ***Indicates p<0.001).
No change in food and water intake was observed in the last consecutive four days of treatment period but slight alterations were seen in food consumption at 1/4 and 1/5 LD50 concentration. The animals were under observation at least once in 30 minute intervals following each treatment. Rats exposed to CYP over a period of 14 days experience ataxia, distress, rolling, and also tremors which were considered as signs of toxicity ranging from a dose of 40 mg/kg body weight (1/9LD50) to 80 mg/kg body weight (1/4.5 LD50) up to the 10th day of treatment following which the symptoms were minimized. There was no evidence of mortality in response to the above mentioned doses.
Before beginning with different systemic studies, some clinically significant biochemical parameters from each system were studied to identify the toxic levels of CYP, From the study, it was so observed that the total RBC count was decreased significantly (p<0.05) from the dose level of 1/9 LD50 to1/4 LD50 in male rats. It was seen that the total leukocyte count was enhanced significantly (p<0.01) at the dose level of 1/9 LD50 but decreased significantly (p<0.01) from the dose level of 1/4.5 LD50 to1/3 LD50 in male rats. No significant changes were shown below 1/9 LD50 dose. The maximum toxic effect of CYP was observed at the 1/4.5LD50 (Table 1) without showing any signs of mortality. From the 1/4 LD50 onwards, the rate of mortality was significantly increased. Similar types of results were observed in the case of hemoglobin percentage (Table 1).
| Table 1: Effect Cypermethrin on Haematological and Hepatic Biomarkers at Different Dose Levels. |
|
RBC Count |
WBC Count |
Hb Percentage |
SGOT |
SGPT |
Hepatic MDA |
| Control |
6±0.577 |
5367±76 |
12.5±0.76 |
54.66±0.881 |
44.83±1.79 |
0.255±0.007 |
| 1/11 LD50 |
6±0.577 |
5367±76 |
12.5±0.76 |
54.66±0.881 |
45.83±1.93 |
0.255±0.007 |
| 1/10 LD50 |
6±0.577 |
5367±76 |
12.5±0.76 |
54.66±0.881 |
45.83±1.93 |
0.255±0.007 |
| 1/9LD50 |
3.833±0.3 a*** |
7233±54
a** |
10.16±0.6 a** |
85.6±0.881 a* |
54.3±1.28 a** |
0.49±0.007 a*** |
| 1/7LD50 |
3.833±0.47a*** |
7117±60 a**
|
10.16±0.6 a** |
87±0.96 a* |
54.3±1.28 a** |
0.49±0.007 a*** |
| 1/5LD50 |
3.333±0.44a*** |
7117±60 a**
|
10.16±0.6 a** |
88±0.96 a* |
60±0.96 a** |
0.721±0.008 a** |
| 1/4.5LD50 |
3±0.36 a*** |
6300±58 a** |
9.16±0.41 a*** |
98.6±0.8 a*** |
73.6±1.15 a*** |
1.24±0.012 a*** |
| 1/4 LD50 |
3±0.36 a*** |
6300±58 a** |
9.16±0.4 a*** |
98.6±0.8 a*** |
73.6±1.11 a*** |
1.24±0.01 a*** |
| 1/3LD50 |
3±0.36 a*** |
6300±58 a** |
9.16±0.4 a*** |
98.6±0.8 a*** |
73.6±1.11 a*** |
1.24±0.008 a*** |
| Results are expressed as Mean±SEM. Data has been analyzed using one way ANOVA. Superscript a Control group versus all other groups (*indicates p<0.05, **indicates p<0.01, ***indicates p<0.001). |
Hepatoxicity
Serum SGOT and SGPT: In Table-1, the effects of CYP on SGOT and SGPT have been exhibited respectively. With an increase in the concentration of CYP, the activity of these two important hepatic enzymes were enhanced significantly (p<0.001) as compared to the control rats. No significant variations were noted below the 1/9 LD50.
Effect on hepatic MDA and glutathione content: In Table 1, hepatic MDA and glutathione content in the control and the CYP-induced experimental groups of the rats have been shown. MDA was seen to be significantly augmented (p<0.01) whereas reduced glutathione content was decreased significantly (p<0.001) with an increase in the dose levels of CYP.
Reproductive Toxicity
Male reproductive toxicity
Effect on sperm count and testicular cholesterol: As shown in figure 3 and 4, CYP resulted in reproductive toxicity as was reflected by the reduced epididymal sperm count (p<0.001), and by an increase in the testicular cholesterol level (p<0.001).
Figure 3: The Effect of Cypermethrin on Sperm Count.
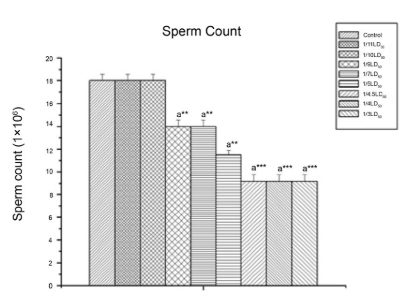 Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
Superscript a Control group versus all other groups (**indicates p<0.01, ***indicates p<0.001).
Figure 4: The Effect of Cypermethrin on Testicular Cholesterol.
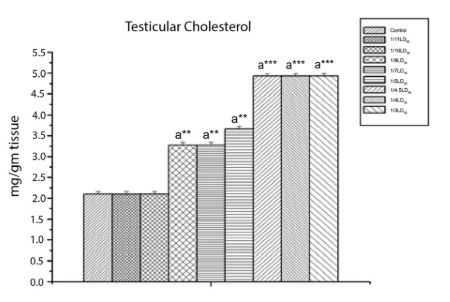
Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA. Superscript a Control group versus all other groups
(***indicates p<0.001).
Effect on testicular Δ5, 3β HSD activity and MDA content: Figure 5 shows that CYP caused a significant decrease (p<0.001) in testicular Δ5,3β HSD activity. Testicular MDA (Figure 6) increased significantly (p<0.01) in response to CYP exposure. From the dose level of 40 mg/kg body wt. (1/9th LD50 dose) onwards, significant changes were detected.
Figure 5: The Effect of Cypermethrin on Testicular 3β HSD Activity.
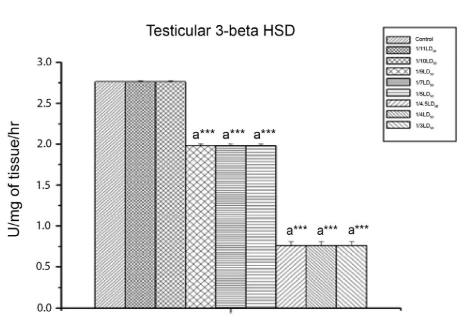 Results are expressed as Mean±SEM. Data analysis has been done by one way ANOVA.
Results are expressed as Mean±SEM. Data analysis has been done by one way ANOVA.
Superscript a Control group versus all other groups (***indicates p<0.001).
Figure 6: The Effect of Cypermethrin on Testicular MDA.
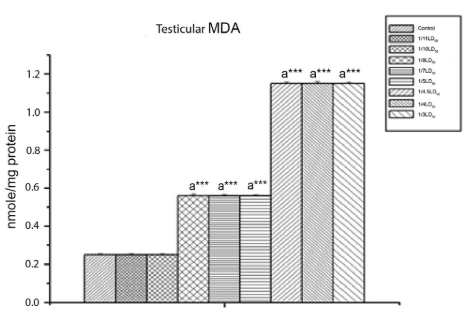 Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
Superscript a Control group versus all other groups (***indicates p<0.001).
Female Reproductive Toxicity
Effect on ovarian cholesterol and 17β HSD: Increased ovarian cholesterol levels and a significant decrease in the ovarian 17β HSD enzyme activity were observed at 1/9LD50 to1/6 LD50 dose level which indicated that CYP induced ovarian toxicity (Table 2).
| Table 2: Effect of Cypermethrin on Ovarian Cholesterol, 17β HSD, MDA and GSH at Different Dose Levels. |
|
Ovarian cholesterol |
Ovarian
17 β HSD |
Ovarian
MDA |
Ovarian
GSH |
| Control |
1.32±0.001 |
2.7±0.006 |
2.65±0.009 |
1.9±0.01 |
| 1/11 LD50 |
1.32±0.001 |
2.708±0.006 |
2.65±0.009 |
1.9±0.01 |
| 1/ 10 LD50 |
1.32±0.001 |
2.708±0.006 |
2.65±0.009 |
1.9±0.01 |
| 1/ 9 LD50 |
1.511±0.0013 a* |
1.2±0.005 a*** |
3.74±0.01 a*** |
1.4±0.009 a*** |
| 1/ 7 LD50 |
1.511±0.0013 a* |
1.2±0.005 a*** |
3.74±0.01 a*** |
1.4±0.009 a*** |
| 1/ 6 LD50 |
1.72±0.0013 a** |
0.531±0.006 a*** |
4.13±0.16 a*** |
1.03±0.007 a*** |
| 1/5 LD50 |
1.72±0.0013 a** |
0.531±0.006 a*** |
4.13±0.16 a*** |
1.03±0.007 a*** |
| 1/4 LD50 |
1.72±0.0013 a** |
0.531±0.006 a*** |
4.13±0.16 a*** |
1.03±0.007 a*** |
| Results are expressed as Mean±SEM. Analysis has been performed using one way ANOVA. Superscript a Control versus all other groups (*indicates p<0.05,**indicates p<0.01, ***indicates p<0.001). |
Effect on ovarian MDA and GSH content: A noticeable dose-dependent increase (p<0.001) in the MDA level and decline in the GSH content were seen in the CYP-induced rat from the 1/9th LD50 dose level onwards.
Experimental dose selection: From these above results for male rats, 1/9 and 1/4.5 LD50 dose and for female rats, 1/9 and 1/6 LD50 dose were considered as effective doses for further studies. As the 1/9 LD50 dose caused minimum significant changes in different measures of systemic biochemical parameters in male rats whereas the effect of 1/11, 1/10 doses did not significantly differ from control and in female rats, the first significant response was observed at the 1/9 LD50 dose. So, in case of male rats, 1/4.5 LD50 dose and for female rats 1/6 LD50 dose were selected as the high doses. Minimum response was recorded at the 1/9 LD50 dose for male and female rats. Therefore, this dose was considered as an effectively low dose for systemic studies.
DISCUSSION
The present study was conducted to investigate the toxic effect of CYP on the different systems of male and female Wistar rats. In toxicity-related studies, body weight is an important parameter for the assessment of organ toxicity. In the current study, body weight was reduced significantly following the oral administration of CYP in both the male and female rats.
The decrease in RBC count after CYP treatment could be due to hemolysis as type II pyrethroid exposure causes hemorrhage and reduction in erythropoiesis.38,39 Some erythrocytes are absorbed by lymphatic vessels due to internal hemorrhages, particularly hemorrhages in the body cavities and the remaining RBCs are lyzed or phagocytosed.40 Various authors have reported similar results in CYP-treated rats,41 sheep,42 rabbit19,43 and goats.44 In the present study, hemoglobin concentration significantly decreased in rats treated with CYP concentrations from 1/9LD50 onwards. Reduction of Hb content could be due to the impaired biosynthesis of heme in the bone marrow.45 Due to the disruptive action of pesticides on the erythropoietic tissue, a decrease in the red blood cell count and hemoglobin content may be observed which could considerably affect the viability of the RBC cells. In addition, the reduction in blood parameters (Hb and RBC) may have been attributed to the hyperactivity of the bone marrow leading to the production of RBCs with impaired integrity which could be easily destroyed in circulation by the reticuloendothelial system. Manna et al41 suggested that a decrease in the RBC count and Hb concentration was indicative of depressed erythropoietin in rats.
The increased WBC counts were noted in CYP-treated groups and it may have been due to the activation of defense and immune system of the body. 19 These findings were also supported by the findings of Yousef et al in sheep,9 and Yousef et al46 in rabbits. This increase in leukocyte count may indicate an activation of the animal’s defense mechanism and immune system. This may also result in an increase in the release of WBC from the bone marrow storage pool into the blood. The primary function of WBCs is to defend the body from foreign bodies, which are generated by leukocytosis and antibody production. Pathological leukocytosis may occur due to exposure to chemicals or acute hemorrhages and hemolysis. Leukocytosis may result due to a resistance developed by the animal towards the localization of the inflammatory response. Another possible cause of leukocytosis may be severe hemorrhages in the liver and lungs.40 This increase may be related to an increase in the lymphocyte percentage.
A significant increase in the SGOT and SGPT levels following CYP treatment at different concentrations in the present study may indicate active utilization of amino acids in energy-yielding metabolic processes like gluconeogenesis. On account of an elevated level of lipid peroxidation products, pyrethroids may induce oxidative stress.47 The observation concerning an increase in the serum glutamate-oxalacetate transaminase (SGOT) and serum glutamate-pyruvate transaminase (SGPT) activities (Table 1) is in agreement with the findings of Yousef et al9,10 and El-Demerdash et al.12 The increase in these serum enzyme activities is suggestive of liver dysfunction. Hence, cellular damage caused by toxic substances is frequently accompanied by increasing cell membrane permeability. The current results showed that CYP led to an increase in MDA and a decrease in the level of GSH which might be attributed to CYP-induced oxidative stress in rat hepatocytes.48
The results have shown reproductive dysfunction following CYP exposure at different doses. The reduction of epididymal sperm count in CYP intoxicated rats drew our attention towards the impairment of spermatogenesis.
The increase in the cholesterol, the main hormone involved in the control of fertility of male animals including rats, level in the testes may be due to its non-utilization. A decrease in the androgenic key enzyme (Δ5, 3β-HSD) activity was highlighted in the CYP-treated group which indicated an inhibition of testicular androgenesis.49 The low steroidogenic enzyme (∆5,3ßHSD) activity also reflected from the above findings indicated the low gonadotrophin levels as the activity of the associated enzymes was regulated by gonadotrophins.49 Oxidative stress defines an imbalance between the production of reactive oxygen species (ROS) and antioxidative defense mechanisms. During pyrethroid metabolism, ROS were generated and caused oxidative stress in intoxicated animals. In oxidative stress, lipid peroxidation occurred due to excessive free radical production and was considered a primary mechanism of cell membrane destruction and cell damage. MDA is the end product of lipid peroxidation. Increased MDA levels in testis suggested that CYP caused testicular damage.50 In the present study, a significant rise in the ovarian cholesterol level of CYP-treated rats indicated less consumption of cholesterol towards the biosynthesis of ovarian steroid hormones. Thus, it resulted in the malfunctioning of ovarian steroidogenic activity of CYP-treated rats. The low steroidogenic activity was also confirmed by the decreased activity of steroidogenic enzymes (17βHSD) which indicated an inhibition of ovarian steroidogenesis.50 Reduction in GSH levels in ovary following CYP treatment may be indicative of oxidative stress, whereas GSH was utilized for the detoxification of reactive toxic substances. As one of the most essential biological molecules, GSH played a key role in the detoxification of the reactive toxic metabolites. Normal cellular function was executed based on a balance between ROS production and antioxidant defense mechanisms existing in the cell.51
CONCLUSION
The present study suggested that CYP intoxication was responsible for hematological, hepatic and gonadal toxicity at 40 mg/kg body weight (1/9 LD50), and 80 mg/kg body weight (1/4.5 LD50) in male rats, and at 34.33 mg/kg body weight (1/9 LD50) and 51.5 mg/kg body weight (1/6 LD50) in female rats.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.

 Results are Expressed as Mean±SEM. The Data has been Analyzed using one way ANOVA.
Results are Expressed as Mean±SEM. The Data has been Analyzed using one way ANOVA. Results are Expressed as Mean±SEM. Data Analysis has been Performed Using One Way ANOVA.
Results are Expressed as Mean±SEM. Data Analysis has been Performed Using One Way ANOVA. Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
 Results are expressed as Mean±SEM. Data analysis has been done by one way ANOVA.
Results are expressed as Mean±SEM. Data analysis has been done by one way ANOVA. Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.
Results are expressed as Mean±SEM. Data analysis has been performed using one way ANOVA.



