INTRODUCTION
Beef and dairy cattle contribute significantly to the economy of a country. The failure of the cows to sustain one-year calving interval (CI), and the failure to become pregnant in heifers account for a high percentage of reduced reproductive efficiency. The CI in cows is influenced by a time lag between calving and the resumption of ovarian activity in the postpartum period.1 A delayed resumption of postpartum ovarian activity in cows is the major limiting factor that prolongs the CI. Although, several studies have indicated abnormal ovarian cyclicity (AOC) and associated ovarian disturbances in cows during the early and pre-service postpartum periods,2 the available data associated with the ovarian activity, and ovarian disorders in cows with delayed conception beyond 90 days postpartum, as well as heifers with delayed age at first calving (>24 months), is limited. Unlike the dairy cows, there is a dearth of available data related to the occurrence of AOCs in beef cattle even during the early postpartum period.
Apart from AOC, ovarian disorders such as follicular and luteal cysts (cystic ovarian diseases (COD) in dairy cows during early and pre-service postpartum period have been incriminated as causes of reproductive failure and economic loss. Results of several studies have indicated a higher frequency of occurrence of ovarian cysts in early postpartum cows that varied from 6 to 30%3 with peak incidences between 14 and 40 days postpartum.4,5,6 Despite such reports on dairy cows, insufficient data is available to validate the occurrence of COD in both dairy and beef cows beyond 90 days postpartum, and heifers with delayed age at first calving. In fact, little is known about COD in beef cows even during the early postpartum period except with a general understanding that COD is a problem that occasionally occurs in beef cows.7 It was, therefore, imperative to investigate the magnitude of occurrence of COD in beef cattle.
Despite an understanding of the incidence and significant effect of AOC and ovarian disorders during the pre-service postpartum period on reproductive performance, there is little evidence to claim that the same problems are the main reasons contributing towards reproductive failure in dairy and beef cows with prolonged postpartum period (>90 days), and heifers with delayed age at first calving. Therefore, the objectives of the present study were to investigate the occurrence of abnormal ovarian cyclicity and associated ovarian disorders such as COD in dairy and beef cattle with reduced reproductive performance based on ultrasonography and P4 profiles.
MATERIALS AND METHODS
The study was carried out at the Universiti Putra Malaysia Beef and Dairy Farms located at the Universiti’s Agricultural Park in Selangor, Malaysia. The average daily temperature of the area is 28 ºC with relative humidity between 70% and 90% annually. Several breeds of bulls (Friesian Sahiwal for dairy cows; Brangus, Brahman, Simmental and local Kedah Kelantan breeds for the beef cows) were used for natural breeding. The bulls were examined for vibriosis and brucellosis and were found to be free from those diseases.
Animals and Management
A total of 102 non-pregnant cows and heifers identified in the dairy and beef herds, were divided into 4 groups according to their breeds [24 Friesian Sahiwal (FRS), 31 Bradford (BRF), 15 Brangus (BR), and 32 Kedah Kelantan (KK)]. Animals were identified based on their records of poor reproductive performance in each herd: delayed age at first calving in heifers with a mean age of 34.6 months (range from 25 to 60 months), and prolonged postpartum open days of more than 90 in cows, with a mean of 11.5 months (range from 3.5 to 60 months), as calculated for the whole group. Before the study commenced, all the identified animals were checked for non-pregnancy status and a clinical diagnosis of health problems were undertaken using palpation per rectum and ultrasonography.
Dairy Group
Of the 24 FRS cows and heifers identified as poor reproductive performers, 20 were cows and 4 were heifers. The average postpartum period of the cows and the delayed first calving age of the heifers were 8.1 months and 34 months, respectively. According to the study of 1 to 5 score by,8 the average body condition score (BCS) for both cows and heifers was 3.4. Seven cows were nonlactating dry cows while 13 were milking cows with calves weaned from the day of calving. The calving rate was 56.6%.
A local commercial feed, dairy cattle pellet, DCP (Federal Flour Mill Sdn., Berhad, Malaysia), which contained 16% crude protein, 15% crude fiber, 13% moisture, 0.5% phosphorus and 0.8-1.5% calcium were fed to each animal in an amount of 5 kg per day, irrespective of the individual cow production status. The cows were allowed to graze in the pasture during the day and housed in roofed stalls in the evening. Water was provided ad libitum. Cows were allowed to run free with the bulls and a bull was used for breeding at a 1: 30 bull-cow ratio.
Beef Group
From the total of 78 beef cattle identified with reproductive problems, 31 were BRF (15 cows and 16 heifers), 15 BR (7 cows and 8 heifers) and 32 KK (23 cows and 9 heifers). The postpartum period of BRF cows ranged from 5 to 28 months (mean=13.4 months), BR cows ranged from 9 to 28 months (mean=19.7 months), and KK cows ranged from 3 to 29 months (mean=4.8 months). The average age of the BRF, BR, and KK heifers was 34.6 months (range from 25 to 60 months), 37.95 months (range from 25 to 60 months), and 31.9 months (25 to 36 months), respectively. The mean BCS for BRF, BR and KK were 3.5, 3.6, and 3.5, respectively based on the 1 to 5 grading scale.9 Unlike the dairy calves which were weaned at the day of calving, the beef calves were allowed to suckle until the age of 7 months. All the KK cows and only 7 of the BRF, and 3 of BR cows in the beef group were with suckling calves while the rest were non-suckling dry cows.
Calving rates for animals in each breed group were: 33.3% (BRF), 43.5% (BR), and 92.4% (KK). The animals in this group were allowed to graze in pastures with palm kernel cake (PKC) supplement of 1-1.5 kg per day per animal. The PKC contains 14% maximum protein, 20% minimum -?, 11.7% moisture, 12% dirt and impurities, and 7% oil. Water was provided ad libitum. Cows were allowed to run free with the bulls and a bull was used at a 1: 30 bull-cow ratio. According to their medical record, all the bulls were diagnosed to be negative for reproductive diseases such as vibriosis and brucellosis, and with no history trichomoniasis.
Blood Sampling, Progesterone Assay and Ovarian Ultrasonography
Blood samples for progesterone (P4 ) analysis were obtained in heparin coated 10 mL sterile vacutainer tubes via a jugular vein puncture twice weekly for about three months (December 2009 to March 2010). Immediately after the sampling tubes were placed in an ice box and transported to the laboratory for processing, these tubes were centrifuged at 2500 rpm for 15 minutes at 4 o C and the separated plasma was transferred to sterile tubes, and stored at -30 o C pending the analysis. Besides blood sampling, the ovarian activity was also monitored for the development of dominant follicles, corpora lutea as well as any cystic ovarian condition, using a B-mode, real-time portable ultrasound scanner equipped with a 5 MHz linear array transducer (SSD 555, Aloka, Japan). Ovarian cysts were detected when there were single or multiple follicles on one or both ovaries with diameter >17 mm that persisted for more than 10 days in the absence of corpus luteum (CL). Further differentiation between various types of ovarian abnormalities such as follicular cysts, luteal cysts, and ovarian inactivity was made based on the plasma P4 profile combined with ultrasonographic findings as described by Mueller.10
Plasma P4 concentrations were measured using a commercial solid-phase radioimmunoassay (RIA) kit (Coat-A-Count; Siemens Medical Solutions Diagnostics, Los Angeles, USA) following the procedure as prescribed by the manufacturer. The observed sensitivity of the kit was 0.02 ng/mL. The intra- and interassay coefficients of variations were 5.52 and 8,21, respectively.
Definition of Ovarian Cycle
A plasma P4 level of 1ng/mL was taken as the threshold value to indicate luteal activity.11 Cows and heifers were classified into the following different groups, based on the characteristics of their P4 profiles, as described previously:12,13,14
1. Normal cycle: Regular cycle throughout the study period and/or those with initial regular cyclicity followed by a high P4 level (>1 ng/mL) being maintained due to pregnancy.
2. Abnormal cycle: Ovarian cycle deviated from the normal cycle which was sub-divided into the following clinical situations:
3. Prolonged luteal phase (PLP): One or more ovarian cycles with luteal activity for more than 20 days. b. Short luteal phase (SLP): One or more luteal phase not exceeding 10 days.
4. Cessation of ovarian cycle: Weak or no luteal activity such that all the P4 levels were below 1 ng/mL for at least 14 days during the study period.
Data Analysis
The occurrence of the total and specific types of AOC for the whole group, and each breed of animals were expressed as a percentage of the total number of animals in the whole group and in each breed, respectively. Since the data recorded was categorical, non-parametric statistical test for more than two independent samples (Kruskal-Wallis test using SPSS V-16.0) was used to test for the presence of significant differences among the beef breeds as well as between the dairy and beef groups. When a statistically significant difference was obtained p<0.05) a separate comparison between any two groups was made using the Mann-Whitney test. The same test was also applied to compare the differences between cows and heifers in the beef group as well as between the beef and dairy cows. The different types of abnormal progesterone profiles observed or AOC, and ultrasonographic observations of ovarian disorders such as ovarian cysts in each breed were represented in percentages.
RESULTS
Abnormal Ovarian Cycles Detected based on Plasma Progesterone Profiles
The frequency of the total and different forms of AOCs in each breed has been shown in Table 1. Out of the total 102 animals (65 cows and 37 heifers), 33.3% showed AOC. The predominant type of AOC being cessation of cyclicity (18.6%) followed by prolonged luteal phases (PLP) and short luteal phases (SLP), which were reported as 9.8% and 4.9%, respectively (Table 1). The remaining 38.2% and 28.4% account for animals with initial regular ovarian cycle followed by pregnancy, and animals with regular cycle but which remained open untill the end of the study respectively (Table 2). When each breed was considered, 37.5% of FRS, 41.9% of BRF, and 53.3% of BR reported AOC (Table 1). The lowest percentage of AOC was observed in the local KK breed (12.5%) and a majority of them were pregnant in the meantime during the study.
| Table 1. Frequency of Abnormal Ovarian Cyclicity and its Type in Dairy (FRS) and Beef (BRF, BR, KK) Cattle Breeds based on P4 Profiles |
|
Breed
|
No. of animals |
No. of animals with AOC (%)
|
Types of AOC (%)
|
|
PLP
|
SLP |
Cessation
|
|
FRS
|
24 |
9 (37.5)a |
6 (25)c |
1(4.2)d |
2 (8.3)e |
| BRF |
31 |
13 (41.9)a |
1 (3.2)c |
2 (6.5)d |
10 (32.3)e
|
| BR |
15 |
8 (53.3)a |
1 (6.7)c |
1 (6.7)d |
6 (40)e
|
|
KK
|
32 |
4 (12.5)ab |
2 (6.3)c |
1 (3.1)d |
1 (3.1)e |
| Total |
102 |
34 (33.3) |
10 (9.8) |
5 (4.9) |
19 (18.6)
|
| Note: Values with different superscripts corresponding to each breed along the column indicate significant difference (p<0.05). |
There were statistically significant differences (p<0.05) in the incidence of AOC when the three breeds were separately compared with the KK breed. However, there was no significant difference (p<0.05) among the three breeds (FRS, BRF, BR) when comparisons were made with each other. Nevertheless, as shown in Table 1, a predominantly higher percentage of cessation of ovarian cycles were found in both BRF (32.3%) and BR (40%) breeds, which altogether accounted for the total 21.8% of AOC found in the beef cows and heifers (Table 2) compared with dairy FRS breeds (8.3%). On the other hand, PLP (25%) was the predominant type of AOC found in FRS breeds compared with BRF (3.2%), BR (6.7%) and KK (6.3%) beef cattle (Table 1). From the total number of beef cows and heifers (BRF+BR+KK), 32.1% (25/78) showed AOC (Table 2). When beef heifers and cows were separately considered, 36.4% (12/33) heifers and 28.9% (13/45) cows showed abnormal ovarian cycles (Table 3). The predominant type of AOC was the cessation of cyclicity (30.3% in heifers and 15.6% in cows), respectively.
| Table 2. Frequency of Normal and the Different Forms of AOC in Dairy Cattle Compared with Beef Cattle Based on P4 Profiles |
|
Group
|
No. of animals |
No. of animals with normal cycles but not pregnant (%) |
No. of animals with normal cycles followed by pregnancy (%) |
No. of animals with abnormal cycles (%) |
Distribution of types of AOC per group (%) |
|
PLP
|
SLP |
Cessation
|
| Dairy |
24 |
8 (33.3)a |
7 (29.2)b |
9 (37.5)c |
6 (25)d |
1 (4.2) e |
2 (8.3)f
|
|
Beef
|
78 |
21 (26.9)a |
32 (41)b |
25 (32.1)c |
4 (5.1)d |
4 (5.1)e |
17 (21.8)f |
| Total |
102 |
29 (28.4) |
39 (38.2) |
34 (33.3)
|
10 (9.8)
|
5 (4.9) |
19 (18.6)
|
| Note: Values with different superscripts along the column show significant difference (p<0.05). |
| Table 3. Frequency of the different Forms of AOC Encountered in Beef Cows and Heifers |
|
Beef Group
|
No. of animals |
No. of animals with AOC (%) |
Distribution of types of AOC per group (%) |
| PLP |
SLP |
Cessation
|
|
Cows
|
45 |
13 (28.9)a |
3 (6.7)b |
3 (6.7)c |
7 (15.6)d |
| Heifers |
33 |
12 (36.4)a |
1 (3)b |
1 (3)c |
10 (30.3)d
|
| Note: Values with different superscripts along the column show significant difference (p<0.05) |
Ovarian Cysts Associated with Abnormal Ovarian Cyclicity
The occurrence of COD which comprises follicular and luteal cysts found in each breed has been shown in Table 4. The highest incidence of COD (30%) was FRS, which was mainly due to luteal cysts that resulted in a prolonged luteal phase of the estrous cycle (Tables 4 and 5). This was followed by 14.3% and 13.3% of COD in the beef cows of BR and BRF, respectively while KK cows showed the lowest incidence (8.7%). On the other hand, a higher incidence of COD in the beef heifers of BRF (31.3%) and BR (25%) was observed compared with the cows of the same breeds as well as the heifers of the FRS and KK breeds (Table 4).
| Table 4. Occurrence of COD among Cows and Heifers in each Breed |
|
Incidence of COD (%)
|
| Breed |
No. of animals (cows + heifers) |
Cows |
Heifers
|
|
FRS
|
24 (20+4) |
30 |
0 |
| BRF |
31 (15+16) |
13.3 |
31.3
|
|
BR
|
15 (7+8) |
14.3 |
25 |
| KK |
32 (23+9) |
8.7 |
0
|
The frequency distribution of the various forms of ovarian abnormalities encountered in each breed was examined using ultrasonography in relation to the types of AOCs based on P4 profiles as has been represented in Table 5. Of the total number of animals (34) with ovarian disorder, 32.4% (11/34) and 29.4% (10/34) of the cases were associated with the presence of follicular cysts and luteal cysts, respectively. The remaining 23.5% (8/34) and 14.5% (5/34) of the cases were attributed to ovarian inactivity and the failure of CL to mature and sustain, respectively (Table 5). Representative ultrasound pictures of ovaries with cystic ovarian disease (follicular cyst and luteal cyst) has been shown in Figures 1 and 2.
Figure 1. Ovary With Luteal Cyst from a Friesian Sahiwal Cow Characterised by Thick Wall (Black Double Arrows) With Septa of Tissues in The Cavity. The Big Arrows Indicate the Demarcation of the Wall of the Cyst from the Ovarian Stroma. Both Pictures Represent the Same Ovary but Scanned at Different Times. The Mean Diameters of the Cysts were 30 mm
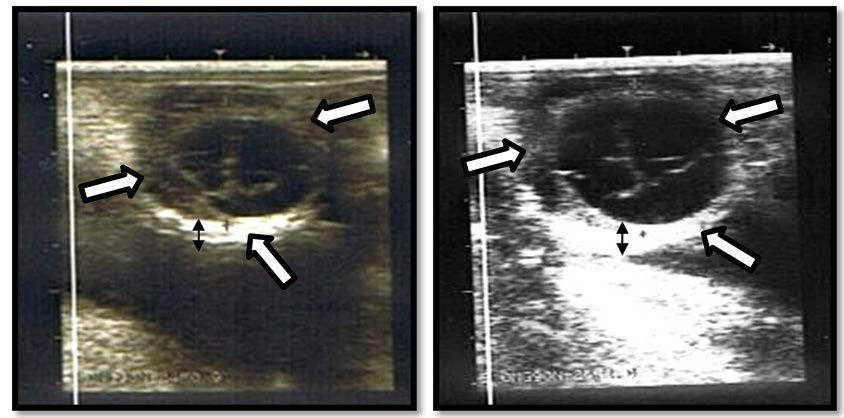
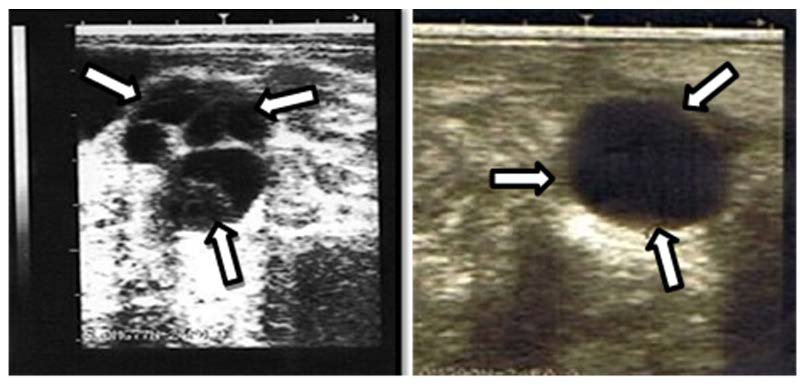
Figure 2. Ovary with a Multi-follicular Cyst from a Brangus Cow (left) and Single Follicular Cyst from a KK Cow (Right). The Arrows Indicate the Thin Wall of the Cyst Surrounding the Cavity was Continued by the Ovarian Stroma. The Mean Diameter of the Single Cyst on the Right Measured 25 mm
| Table 5. Frequency Distribution of Ovarian Disorder Types Diagnosed by Ultrasonography Together with AOC Types Identified Based on P4 Profile |
|
Types of ovarian disorder
|
|
Types of AOC
|
Frequency |
Follicular cyst |
Luteal cyst |
Inactive ovary |
Failure to sustain CL
|
|
Cessation
|
19 |
11 |
– |
8 |
– |
| PLP |
10 |
– |
10 |
– |
–
|
|
SLP
|
5 |
– |
– |
– |
5 |
| Total |
34 |
11 |
10 |
8 |
5
|
Among the different types of AOC observed, cessation of ovarian cyclicity (19/34) represented the predominant type of ovarian disturbance, which according to the ultrasound finding was due to follicular cysts (11/19) and ovarian inactivity (8/19) (Table 5). This was mainly encountered in both BRF and BR beef breeds. However, PLP, which accounted for 29.4% (10/34) of the total AOC observed was the most important problem encountered in dairy FRS breed due to luteal cysts (Tables 1 and 5). The P4 profile that characterised SLP was associated with the failure to sustain a CL (Table 5). Luteal cysts primarily accounted for a major type of COD in the dairy FRS cows, while follicular cysts were observed in the beef breeds in this study.
DISCUSSIONS
In order to achieve an optimal calving interval of 1 year in a dairy and beef farming enterprise, the cycle of the cows should resume early in the postpartum period and they should be able to breed within 65 days after calving.2,14 However, this has been reportedly affected adversely by an increased incidence of ovarian disturbances in the immediate postpartum period mainly in dairy cows1,2,15,16 and prolonged postpartum acyclicity in beef cows.17 According to Opsomer et al2, abnormal P4 profiles were reported in 46.5% of dairy cows studied. Of the 46.5% abnormal profiles, PLP, cessation of cyclicity and SLP accounted for 20%, 3%, and 0.5%, respectively. In the present study, in the dairy cattle alone (>90 days postpartum), occurrence of 37.5% abnormal P4 profiles was recorded (Table 2), which was comparable with the findings of Shrestha et al.1 However, there was a relatively higher incidence of PLP (25% vs. 20%), and cessation of cyclicity (8.3% vs. 3%). The high occurrence of PLP in this study was in agreement with the findings of Petersson et al12 who reported PLP as the main type of atypical P4 profile in Swedish dairy cows (<60 days postpartum). In another study,16 a similar occurrence for cessation of ovarian cycle (10.5%) was reported in dairy cows less than 60 days postpartum, but with a low incidence of PLP (0.5%). The observations were similar in this study compared with the previous studies12,16 for PLP and cessation of ovarian cycle, respectively, reported in the pre-service postpartum period (<60 days) which might have indicated the existence of problems in cows having a postpartum length of more than 90 days as a continuation of the pre-service period.>60 days postpartum). In another study,16 a similar occurrence for cessation of ovarian cycle (10.5%) was reported in dairy cows less than 60 days postpartum, but with a low incidence of PLP (0.5%). The observations were similar in this study compared with the previous studies12,16 for PLP and cessation of ovarian cycle, respectively, reported in the pre-service postpartum period (<60 days) which might have indicated the existence of problems in cows having a postpartum length of more than 90 days as a continuation of the pre-service period.
Abnormal luteal function in lactating cows have been associated with reduced pregnancy rate, whereby cows with normal P4 profiles showed significantly higher pregnancy rates relative to cows with abnormal profiles (87% vs. 33%) following AI.18 Moreover, a significantly higher AI submission rate, conception rate, and pregnancy rate between 44 and 100 days postpartum were reported in dairy cows with a normal ovarian cycle in the first 44 days postpartum than those with prolonged luteal phases.1,15 This might confirm the high percentage of abnormal luteal activity, mainly PLP (25%), in dairy cows in the present study, to be a major cause for the reduction in the fertility observed in the dairy group.
During pregnancy, bovine conceptus produces a signal about 14 days after fertilization which results in prolongation of the corpus luteum. However, if an embryo dies at 14 or 15 days of gestation (early embryonic death), the cow will return to estrus at a normal interval and the cycle remains regular.14 In the current study, the P4 profiles of 28.4% of the total number of cows and heifers showed regular ovarian cycles, but still remained open in the presence of bulls (Table 2). This might indicate that these animals were suffering from early embryonic mortality which was a major cause of reproductive failure in cows,19 apart from the bulls’ fertility status. On the other hand, if an embryo dies between days 16 and 42 post-breeding, the luteal activity is displayed as a prolonged luteal phase based on the progesterone profile.14 According to heat detection and mating records in the dairy group made on opportunistic basis, prolonged luteal phases between successive matings observed in some animals were most likely due to the late embryonic mortality.
Comparison between dairy and beef cattle showed a difference in the rate of incidence expressed as a percentage and types of AOC, though not significant (Table 2). Compared with beef cattle, dairy cattle in this study showed a higher percentage of AOC (37.5% vs. 32.1%). The predominant type of AOC observed in dairy cows was PLP (25%), while cessation of cyclicity was predominantly found in beef cattle (21.8%). The differences may have been due to the effect of suckling or cow-calf interaction which was known to cause delayed resumption of ovarian activity and an estrus period in beef cows compared to dairy cows.17 Moreover, nutritional and genetic differences may have also contributed to the variation in incidence and types of AOC between the dairy and beef cattle. When beef heifers and cows were separately considered, beef heifers presented a comparable incidence of AOC with beef cows (36.4% vs. 28.9%) as has been shown in Table 3. The predominant type of AOC was marked by cessation of cyclicity (30.3% in heifers and 15.6% in cows). Beef heifers have been reported to have lower fertilization rates and reproductive failure equally attributable to both fertilization failure and early embryonic loss prior to day 8 of the gestation period like in beef cows.
The local KK cattle in this study showed a statistically significant difference from the other three breeds, in the incidence of AOC and its types; with a low occurrence of AOC and a majority of they were observed to be pregnant by the end of the study (Table 1). This may not be surprising as these cows were identified from a herd with a record of high calving rates (92%), compared with the other breeds: dairy FRS (56.6%), BRF (33.3%), and BR (43.5%). This might generally indicate that genetic differences between breeds in the incidence and types of AOC can result in differences in fertility parameters such as conception rate.20 In addition, adaptation differences to the hot and humid tropical environment may account for the large difference between the local KK and the other three breeds. Under the hot and humid conditions, despite high rainfall that produces more forage, high humidity, and temperature, could cause stress and affect their reproductive functions by modifying the endocrine balance and altering the oviductal uterine environment that delays or interrupts embryo development.20 According to,21 dairy cows with COD have been reported to have increased heat shock protein (Hsp27) expressions theca cells in relation to tertiary follicles of normal cycling cows, indicating the possible link between COD occurrence and heat stress in cattle. Zebu cows (Bos indicus) and their crossbreeds show lower levels of milk production and better adaptation to the adverse tropical environment, and hence show lesser effects on the postpartum body condition but a higher reproductive efficiency than the predominantly Bos taurus cows.20
Variable incidence rates of ovarian cysts that ranged from 6% to 30% in postpartum dairy cows mainly during the pre-service period in temperate regions have been reported.3 In the present study, an incidence of 30% COD in dairy FRS cows more than 90 days postpartum has been recorded which was on the upper side of the range of 6 to 30% reported earlier in other areas.3 The reported data was also higher than the data reported in the tropics (4%).22 According to Mujuni et al22 in their longitudinal study until 150 days postpartum, have reported a higher incidence of COD during the 60 to 90 days postpartum unlike other studies6,23 that have indicated a higher occurrence between 30 and 60 days postpartum. Although sufficient data was not available before 90 days postpartum in the cows in the present study, it was apparent that the observed incidence of COD was much higher than the earlier reports in cows before 90 days postpartum. The high incidence of COD observed in this study may have been because the cows were identified from a herd with fertility problems as well as due to the hot humid tropical environment and poor management. A higher risk of developing ovarian cysts were reported in cows which were calving during the warm season due to heat stress than those calving in the cool season, in the previous studies.6,24 In contrast to earlier studies that reported follicular cysts as a more common problem than luteal cysts in dairy cows,7,25 a majority of the total 30% ovarian cysts encountered in the present study were luteal cysts (Table 3). This may have been due to the difference in the phase of the postpartum period of the cows studied (i.e., more than 90 days), which had probably given enough time for follicular cysts to luteinize and form luteal cysts.
Despite ample information being available on the incidence of ovarian cysts in dairy cows, there is a lack of adequate data supporting this observation in beef cows and heifers. It has been generally stated that COD is a common problem in dairy cows but occasionally detected in beef heifers and cows.7 However, the current study showed that COD is also an important problem in beef cows as well as heifers. An incidence of 13.3% and 14.3% of COD in the beef cows of BRF and BR breeds, respectively were recorded while KK cows showed the lowest incidence (8.7%). In addition, a higher incidence of COD was observed in the BRF beef heifers (31.3%), which was mainly due to follicular cysts and BR heifers (25%) compared with cows of the same breeds as well as the FRS and KK heifer breeds. All the incidence rates for COD encountered in beef heifers and cows in this study were within the range of COD incidence (6% to 30%) which was reported previously in dairy cows except for BRF heifers in which the reported cases was slightly higher (31.3%). Although, it is not possible to compare other beef cattle elsewhere due to the inadequate information being available for studies in beef cattle, a comparison of the reports in dairy cows showed that COD was also a serious problem that warranted more research and attention in beef cattle, unlike the reports presented by Kesler et al7 in which COD was considered as an occasional problem. The reason s underlying the difference between the incidence of COD in dairy cows, beef cows, and heifers is not clear. However, genetics may have played a role for the observed difference among the breeds. A direct relationship between genetics and COD has been indicated by a reduced clinical incidence of ovarian cysts from 10.8 to 3.0% in Sweden as a result of genetic selection.7 Moreover, a difference in adaptation to the hot humid tropical weather may also have contributed to the variation especially favouring the local KK cows that showed the lowest incidence. An impaired final follicular maturation and steroidogenesis under heat stress has been reported26 that can lead to ovarian cyst formation. Recent studies have explained possible involvement of altered mRNA expression and activities of the Matrix Metalloproteinases and their Inhibitors,27 as well as impaired insulin signaling pathway in ovarian follicles in bovine COD development.28 Increased milk yield in dairy cows has also been suggested to increase the risk of developing ovarian cysts26 which could be another possible factor for its higher clinical incidence in dairy cows.
Postpartum cows have been reported in previous studies with peak incidences between 14 and 40 days postpartum.24 Other studies have also reported that up to 70% of the ovarian cysts occur between days 16 and 50 after calving, with the highest occurrence between days 30 and 40 after calving.4,5 The periods before day 16 and after day 50 post-calving shows the lowest incidence of COD.23 The current data showed that COD was not only a problem in the early postpartum period of dairy cows but also in the dairy and beef cows delayed for more than 90 days postpartum as well as in beef heifers due to delayed age at first calving. This indicated that COD existed as a serious cause of reproductive failure and economic loss by prolonging the calving interval in cows as well as the age at first calving in heifers. A delay in mean calving to conception by 19-73 days with nearly two more services required per conception29,30 as well as a prolonged calving interval by 40 to 50 days in COD animals compared with unaffected herd mates may have been reported in the earlier studies.10,31,32 The chance of spontaneous recovery for cysts that develop in early postpartum is more than 50% while for cysts after 50 days postpartum, the recovery without treatment is reduced to 20% and waiting for spontaneous recovery during the breeding period extends further the calving interval.26 Similarly for the COD that is detected in cows in this study, as they are beyond 90 days postpartum with fewer chances of spontaneous recovery, treatment of the disorder should be considered to shorten the CI and minimize economic losses.
Follicular cysts are characterised by low plasma P4 concentrations (<1 ng/Ml) unlike the luteal cysts which are accompanied by high P4 levels.31Thus, the use of P4 profile helps to identify the two types of ovarian cysts. More accurate differentiation and the distinction between the two cysts is achieved by using transrectal ultrasonography. The use of ultrasound was a necessity to differentiate between follicular cysts and ovarian inactivity, where there is a low P4 level in both cases that was otherwise difficult to differentiate by using P4 analysis only. Therefore, the combined use of P4 analysis and ultrasonography is useful to accurately differentiate the different types of ovarian abnormalities such as follicular cyst, luteal cyst, and ovarian inactivity. The ovarian inactivity was characterised by the presence of little (<5 mm diameter follicles) or no follicular development while the follicular cyst was characterised by the presence of a fluid filled thin walled cavity when examined with ultrasonography. Follicular cysts in this study appeared both as single and polycystic forms with diameters less than 25 mm, which was against the traditional definition of follicular cysts that sets minimum diameter of 25 mm7 but in agreement with later reports.10,32
CONCLUSIONS
The present study demonstrated a high incidence of AOC and COD as a cause of reproductive failure in dairy and beef cows (>90 days postpartum), which is a well-known ovarian disturbance during the preservice postpartum period especially in dairy cows. The incidences were higher for Braford and Brangus breeds which have not been reported elsewhere. The study also revealed PLP as a predominant type of AOC in dairy cows of FRS and cessation of ovarian cyclicity in beef cattles of both BRF and BR breeds, but lower incidence of AOC was observed in the local KK cows and heifers. The PLP in dairy cows was due to the presence of luteal cysts in the ovary which accounted for the most frequent form of COD while cessation of ovarian cyclicity in the beef groups was mainly due to follicular cysts and ovarian inactivity. In other words, luteal cysts in dairy FRS and follicular cysts in beef cows and heifers represented the most frequent type of ovarian cysts encountered. These might indicate the impact of differences in breed, age, and the primary risk factors for AOC and associated ovarian disorders in dairy and beef cows that remained open beyond 90 days postpartum and heifers with delayed age at first calving. This indirectly validates the need to undertake further research in order to identify the risk factors in both dairy and beef cows that remain open beyond 90 days postpartum and in heifers with delayed age at first calving for the correct approach to be applied in preventing the problem. The use of ovarian ultrasonography in this study has enabled the identification of different types of ovarian disorders associated with abnormal plasma P4 profiles.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







