INTRODUCTION
Maize is the third most important cereal grown in the world after wheat and rice. In several countries of West and Central Africa, it is the most cultivated cereal and one of the most consumed, particularly in the countries of the Gulf of Guinea.1 In Togo, maize is a staple food and its production represents at least 50% of that of all cereals (sorghum, rice and millet). The seed is eaten directly as immature cobs that are roasted, boiled, or processed into flour and semolina.2
The food use of maize is developed in both rural and urban areas of Togo, mainly due to the availability of the product in various forms and to the fact that people have been eating habits for decades.2
However, like other crops, maize is exposed to several fungal pathogens, including Ascomycetes of the genus Aspergillus, during cultivation and storage. The aflatoxins secreted by the toxigenic strains of the flavi section are, indeed, formidable mycotoxins that can contaminate maize seeds, altering their physical, nutritional and organoleptic quality.3 These mycotoxins are secondary metabolites that have been shown to be harmful to human and animal health.4
Of all the aflatoxins produced in nature (B1, B2, G1, G2), aflatoxin B1 (AFB1) is the most common in food and has the most potent genotoxic and carcinogenic properties (European Food Safety Authority (EFSA)).5 Initial observations have shown that, at high concentrations, these aflatoxins are violent poisons, and when administered in small doses to laboratory animals, they produce liver cancer.6 More recent studies have confirmed that they can also cause respiratory tract cancer,7 Kwashiorkor8,9,10 and stunted growth especially in countries where malnutrition is prevalent.11
Togo, like other countries in the West African sub-region, does not have its own phytosanitary legislation in relation to acceptable levels of aflatoxin in foodstuffs. The country is in fact subject to the standards of the Codex Alimentarius which postulates a threshold of acceptability of 10 µg/kg for maize.
This study was conducted with the objective of assessing the levels of aflatoxin contamination of maize produced in the two regions (region Maritime and regions des Plateaux) and sold in the markets, in order to compare them with reference standards of the European Union, the Codex Alimentarius and the United States of America which are respectively 4 µg/kg, 10 µg/kg and 20 µg/kg.
MATERIALS AND METHODS
Study Site
Our maize sampling campaign took place from July 20 to August 31, 2018. It took place in two regions in the south of the country, region Maritime and region des Plateaux. Previous studies have shown that these two regions have the largest maize fields and serve the rest of the country with this cereal.2
Sampling
In order to obtain a representative sample, we conducted a basic random survey. We went to each of the subdivisions of the two regions and then we targeted the market days of the main towns that welcome the saleswomen from the surrounding villages. We then proceeded to sample 750 g to 1 kg of maize from several saleswomen. In each region a total of 25 samples were taken. Each sample was placed in a paper bag. A code indicating the coordinates of the sample was stuck on every sample.
Saleswomen were interviewed during the survey and general information such as storage methods of the harvested products was collected. On the field we found that the bags were overlayed horizontally on the ground before being covered by tarpaulins at nightfall. The biggest saleswomen had stores where they stored the bags of maize in rooms without ventilation where there was a lot of humidity and heat. All of the women surveyed said that they do not use any products to preserve their grain.
Dosage of Aflatoxin with VICAM
The VICAM method was used for the analysis. A 50 g sub-sample of each finely ground corn sample was placed in one with an Erlenmeyer flask, 5 g of industrial salt (NaCl) added to facilitate filtration and 100 ml of 80% methanol. The flask closed with a parafilm is placed for 30-minutes on an agitatorat 250 rpm to obtain a homogeneous mixture and release the aflatoxin molecules contained in the maize. The mixture is filtered twice. Firstly using filter paper with a mesh size of 0.5 µm, then using 0.22 µm mesh paper to be able to retain the finer residues.
After filtration the purification was carried out by passing through an immunoaffinity column. The separation of aflatoxin from the filtrate is based on the antigen-antibody reaction for the selective purification of mycotoxins from food extracts. The principle is that the analyte present in the sample is retained by a specific antibody attached to the stationary phase of the column. Interfering compounds not recognized by the antibody are not retained. Purification consists of pouring 2 ml of the solution into a syringe connected to a VICAM (AflaTest) test column. These columns allow the binding of aflatoxin molecules by antibodies located on the stationary phase and the release of impurities. The system is rinsed with 5 ml of distilled water. The release of the analyte during the elution phase is achieved by breaking the bonds with the antibody. After the fixation phase 1 ml of 100% methanol is added to the syringe for elution. The eluate is collected in a microtube.
Detection and quantification is done by adding 1ml of developer (whose composition is not given by the manufacturer) to the purified sample to increase the fluorescence of the aflatoxin molecules. The solution is then subjected to electrical agitation. The tube is cleaned to remove any residue from the surrounding area and inserted into the VICAM fluorometer to take a reading to determine the concentration of aflatoxin in µg/kg.
Data Analysis
The results obtained were exploited using a dynamic cross-tabulation of an Excel sheet.
RESULTS
All samples taken in Region Maritime tested positive for aflatoxin with a maximum level of 450 µg/kg and a minimum level of 1.4µg/kg.
In the second region (Region des Plateaux), there was 100% aflatoxin contamination of the samples. The maximum level noted is 1600 µg/kg and the minimum is 0.17 µg/kg.
DISCUSSION
Our study is a preliminary work whose objective was to measure the total aflatoxins contained in the maize produced in Togo and consumed by millions of Togolese.
Assay results showed a wide variability in aflatoxin levels ranging from a minimum of 0.17 ppb to a maximum of 1600 ppb (Figure 1). The grades obtained were very high for many samples and some even exceeded our expectations, especially sample 25 from Region des Plateaux with a contamination peak of 1600 µg/kg. Samples from Region des Plateaux show higher contamination with a median of 25 µg/kg compared to a median of 9.2µg/kg in Region Maritime. This is not surprising considering the fact that corn has, by far, the highest reported levels of aflatoxin compared to rice, which has lower levels of contamination.12
Figure 1. Aflatoxin Assay Results for the Maize Samples Studied
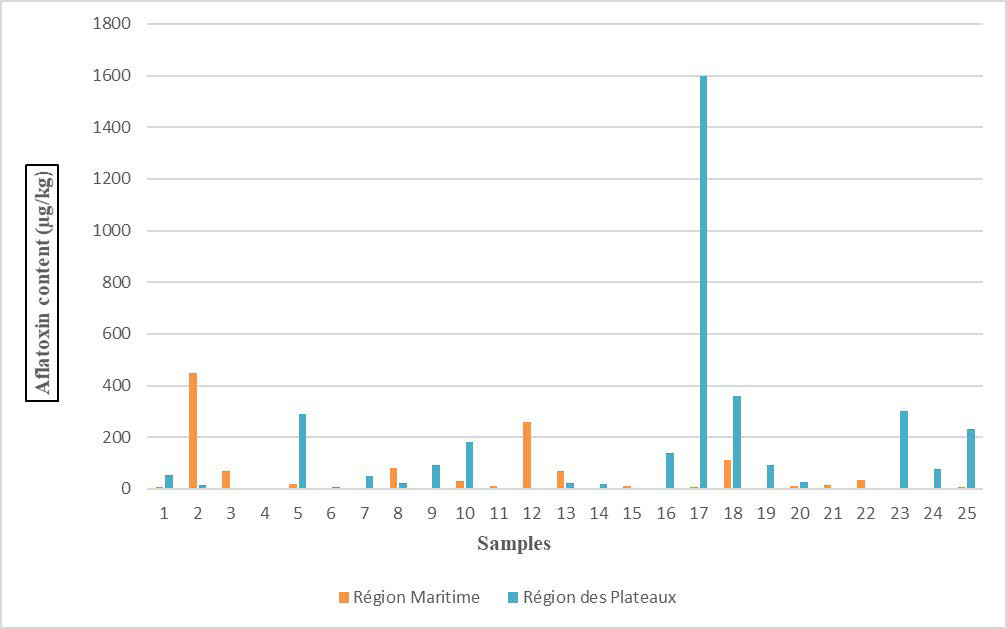
These results are not consistent with those of Diedhiou et al13 during his study on Aspergillus colonization and aflatoxin contamination of maize grains in two agro-ecological zones of Senegal, namely the groundnut basin and the eastern SenegalCasamance zone. During the study they analyzed 25 corn samples. Aflatoxin levels in samples from the East Senegal-Casamance zone ranged from 0 to 1.7 µg/kg. Samples from the groundnut basin had slightly higher levels with an average of 15.9 µg/kg.
The origin of these high-levels could be multiple. It could be due to seed contaminated with Aspergillus flavus and Aspergillus parasiticus, humid and hot climate, contaminated soil prior to cultivation, poor storage and/or warehousing conditions leading to mould growth following aflatoxin biosynthesis, or for unknown reasons.
The field observations allowed us to identify poor storage conditions for the grain. Indeed, bags of maize are often stored in the open air covered by a tarpaulin at nightfall, or in stores where it is excessively hot because there is no ventilation. All of the women surveyed revealed that they do not use any products to preserve the cereal, to control any mould infestation nor to control insects. The lack of means of prevention makes the cereal even more vulnerable and more exposed to any asparagus contamination. These poor storage conditions found in the field are probable sources of proliferation of the contaminant.
The very high-levels obtained allow us to deduce the health danger to which the Togolese population is exposed considering the risks posed by the repeated ingestion of highly contaminated products. Several studies have demonstrated carcinogenicity, and all other damage caused by ingestion or inhalation of aflatoxin molecules.7-16 We can add to that, the stability of the toxin up to temperatures around 300 °C, which are not reached with our cooking methods.17
The first victims are the children because they consume corn very early. In Togo, post-weaning meals are essentially made up of maize-meal-based porridges. This confirms the results of Egal et al18 which showed that exposure of children (9-months to 5-years) to aflatoxin in Togo was linked to high maize consumption. This exposure may cause stunted growth and kwashiorkor.8,9,10
The results obtained on the distribution of aflatoxin content in each region showed that very few samples are conformed to the different standards mentioned above which are 4 ppb for the European Union, 10 ppb for the Codex Alimentarius and 20 ppb for the United States of America (Figure 2). Togo, therefore, seems ineligible on the international market, since the level of contamination of these cereals does not allow it to trade with the member states of these various institutions.
Figure 2. Percentage of Aflatoxin Content of Corn Samples in Relation to Some Regulatory Aflatoxin Standards
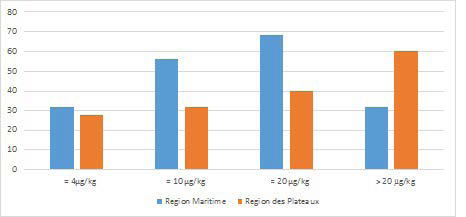
The high presence of aflatoxinin maize samples from these two regions is a real public health issue in relation to the exposure of Togolese populations. In addition to this, the frequency and quantity of maize consumption in Togo is also high, as it should be remembered that the production areas are also areas of high food consumption in relation to the ethnic composition and eating habits.
The establishment of consumer protection standards will take into account the level of contamination of aflatoxincarrying products and the frequency of their consumption. In fact, the standards are function of the frequency of consumption of food likely to be contaminated. In Togo, given to the frequency of consumption of maize and other products likely to be contaminated, the standard should be lower than that of the European Union. This makes the results of our study even more alarming. Togo should then have its own standards.
CONCLUSION
Our study showed a high-level of aflatoxin contamination in corn. This contamination is due to the presence of fungi of the genus Aspergillus from the Flavisection such as Aspergillus flavus and Aspergillus parasiticus. Samples from Region des Plateaux were the most contaminated and their levels were well above the international standards with which they were compared. This shows the health danger to which Togolese consumers are exposed. Because of the high consumption of this cereal, it is then imperative to establish institutions to monitor the maize and its derivatives injected into the market in order to preserve the health of the population.
DISCLOSURE
The authors declare that this study is approved by the Institutional Review Board (IRB).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







