1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394-424. doi: 10.3322/caac.21492
2. Butler HJ, Ashton L, Bird B, et al. Using Raman spectroscopy to characterize biological materials. Nat Protoc. 2016; 11(4): 664- 687. doi: 10.1038/nprot.2016.036
3. Harrison JP, Berry D. Vibrational spectroscopy for imaging single microbial cells in complex biological samples. Front Microbiol. 2017; 8: 675. doi: 10.3389/fmicb.2017.00675
4. Kong K, Kendall C, Stone N, Notingher I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv Drug Deliv Rev. 2015; 89: 121-134. doi: 10.1016/j.addr.2015.03.009
5. Ralbovsky N, Lednev IK. Raman hyperspectroscopy shows promise for diagnosis of Alzheimer’s. Biophotonics International. 2018; 25(4): 33-37.
6. Ryzhikova E, Kazakov O, Halamkova L, et al. Raman spectroscopy of blood serum for Alzheimer’s disease diagnostics: Specificity relative to other types of dementia. J Biophotonics. 2015; 8(7): 584-596. doi: 10.1002/jbio.201400060
7. Meksiarun P, Ishigaki M, Huck-Pezzei VAC, et al. Comparison of multivariate analysis methods for extracting the paraffin component from the paraffin-embedded cancer tissue spectra for Raman imaging. Scientific Reports. 2017; 7: 44890. doi: 10.1038/ srep44890
8. Cals FL, Koljenović S, Hardillo JA, Baatenburg de Jong RJ, Bakker Schut TC, Puppels GJ. Development and validation of Raman spectroscopic classification models to discriminate tongue squamous cell carcinoma from non-tumorous tissue. Oral Oncol. 2016; 60: 41-47. doi: 10.1016/j.oraloncology.2016.06.012
9. Krafft C, Dietzek B, Popp J. Raman and CARS microspectroscopy of cells and tissues. Analyst. 2009; 134(6): 1046-1057. doi: 10.1039/b822354h
10. Kendall C, Isabelle M, Bazant-Hegemark F, et al. Vibrational spectroscopy: A clinical tool for cancer diagnostics. Analyst. 2009; 134(6): 1029-1045. doi: 10.1039/b822130h
11. Krafft C, Knetschke T, Siegner A, Funk RH, Salzer R. Mapping of single cells by near infrared Raman microspectroscopy. Vibrational Spectroscopy. 2003; 32(1): 75-83. doi: 10.1016/S0924-2031(03)00049-3
12. Rau JV, Graziani V, Fosca M, et al. RAMAN spectroscopy imaging improves the diagnosis of papillary thyroid carcinoma. Scientific Reports. 2016; 6: 35117. doi: 10.1038/srep35117
13. Lloyd GR, Orr LE, Christie-Brown J, et al. Discrimination between benign, primary and secondary malignancies in lymph nodes from the head and neck utilising Raman spectroscopy and multivariate analysis. Analyst. 2013; 138(14): 3900-3908. doi: 10.1039/c2an36579k
14. Shaikh R, Prabitha VG, Dora TK, et al. A comparative evaluation of diffuse reflectance and Raman spectroscopy in the detec tion of cervical cancer. Journal of Biophotonics. 2017; 10(2): 242- 252.doi: 10.1002/jbio.201500248
15. Bakker Schut TC, Caspers PJ, Puppels GJ, et al. Discriminating basal cell carcinoma from its surrounding tissue by Raman spectroscopy. J Invest Dermatol. 2002; 119(1): 64-69. doi: 10.1046/j.15231747.2002.01807.x
16. Petersen D, Mavarani L, Niedieker D, et al. Virtual staining of colon cancer tissue by label-free Raman micro-spectroscopy. Analyst. 2017; 142(8): 1207-1215. doi: 10.1039/c6an02072k
17. Han B, Du Y, Fu T, et al. Differences and relationships between normal and atypical ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma tissues in the breast based on Raman spectroscopy. Appl Spectrosc. 2017; 71(2): 300-307. doi: 10.1177/0003702816681009
18. Surmacki J, Musial J, Kordek R, Abramczyk H. Raman imaging at biological interfaces: Applications in breast cancer diagnosis. Mol Cancer. 2013; 12(1): 48. doi: 10.1186/1476-4598-12-48
19. Surmacki J, Brozek-Pluska B, Kordek R, Abramczyk H. The lipid-reactive oxygen species phenotype of breast cancer. Raman spectroscopy and mapping, PCA and PLSDA for invasive ductal carcinoma and invasive lobular carcinoma. Molecular tumorigenic mechanisms beyond Warburg effect. Analyst. 2015; 140(7): 2121- 2133. doi: 10.1039/c4an01876a
20. López-Peña I, Leigh BS, Schlamadinger DE, Kim JE. Insights into protein structure and dynamics by ultraviolet and visible resonance Raman spectroscopy. Biochemistry. 2015; 54(31): 4770- 4783. doi: 10.1021/acs.biochem.5b00514
21. Smith E, Dent G. Chapter 4: Resonance Raman Scattering. In: Modern Raman Spectroscopy: A Practical Approach. Hoboken, New Jersey, United States: John Wiley & Sons; 2005. 93-112.doi: 10.1002/0470011831.ch5
22. Colaianni SM, Aubard J, Hansen SH, Nielsen OF. Raman spectroscopic studies of some biochemically relevant molecules. Vibrational Spectroscopy. 1995; 9(1): 111-120. doi: 10.1016/0924- 2031(94)00066-P
23. Bocklitz TW, Salah FS, Vogler N, et al. Pseudo-HE images derived from CARS/TPEF/SHG multimodal imaging in combination with Raman-spectroscopy as a pathological screening tool. BMC Cancer. 2016; 16(1): 534. doi: 10.1186/s12885-016-2520-x
24. Evans CL, Potma EO, Puoris’ haag M, Côté D, Lin CP, Xie XS. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc Natl Acad Sci U S A. 2005; 102(46): 16807-16812. doi: 10.1073/pnas.0508282102
25. Camp Jr CH, Jong Lee Y, Heddleston JM, et al. High-speed coherent Raman fingerprint imaging of biological tissues. Nat Photonics. 2014; 8(8): 627-634. doi: 10.1038/nphoton.2014.145
26. Kumar S, Kamali T, Levitte JM, et al. Single-pulse CARS based multimodal nonlinear optical microscope for bioimaging. Opt Express. 2015; 23(10): 13082-13098. doi: 10.1364/OE.23.013082
27. Popov K, Pegoraro A, Stolow A, Ramunno L. Image formation in CARS and SRS: Effect of an inhomogeneous nonresonant background medium. Opt Lett. 2012; 37(4): 473-475. doi: 10.1364/OL.37.000473
28. Potma EO, Xie XS. CARS microscopy for biology and medicine. Optics and Photonics News. 2004; 15(11): 40-45. doi: 10.1364/OPN.15.11.000040
29. Vartiainen EM, Rinia HA, Müller M, Bonn M. Direct extraction of Raman line-shapes from congested CARS spectra. Opt Express. 2006; 14(8): 3622-3630. doi: 10.1364/OE.14.003622
30. McVey A, Crain J. Nonlinear optical methods for cellular imaging and localization. Methods. 2014; 68(2): 371-377. doi: 10.1016/j.ymeth.2014.03.002
31. Freudiger CW, Min W, Saar BG, et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science. 2008; 322(5909): 1857-1861. doi: 10.1126/science.1165758
32. Francis A, Berry K, Chen Y, Figueroa B, Fu D. Label-free pathology by spectrally sliced femtosecond stimulated Raman scattering (SRS) microscopy. PLoS One. 2017; 12(5): e0178750. doi: 10.1371/journal.pone.0178750
33. Shen Y, Xu F, Wei L, Hu F, Min W. Live-cell quantitative imaging of proteome degradation by stimulated raman scattering. Angew Chem Int Ed Engl. 2014; 53(22): 5596-5599. doi: 10.1002/ anie.201310725
34. Zhang D, Wang P, Slipchenko MN, Cheng J-X. Fast vibrational imaging of single cells and tissues by stimulated Raman scattering microscopy. Acc Chem Res. 2014; 47(8): 2282-2290. doi: 10.1021/ar400331q
35. Long DA. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules. Hoboken, New Jersey, USA: John Wiley & Sons, Ltd.; 2002.doi: 10.1002/0470845767.ch4
36. Asher SA. UV resonance Raman studies of molecular structure and dynamics: Applications in physical and biophysical chemistry. Annu Rev Phys Chem. 1988; 39(1): 537-588. doi: 10.1146/annurev.pc.39.100188.002541
37. Carey PR. Molecular Biology: Biochemical Applications of Raman and Resonance Raman Spectroscopies. New York, USA: Academic Press. 1982.
38. Huang C-Y, Balakrishnan G, Spiro TG. Protein secondary structure from deep-UV resonance Raman spectroscopy. Journal of Raman Spectroscopy. 2006; 37(1-3): 277-282. doi: 10.1002/ jrs.1440
39. Shashilov VA, Sikirzhytski V, Popova LA, Lednev IK. Quantitative methods for structural characterization of proteins based on deep UV resonance Raman spectroscopy. Methods. 2010; 52(1): 23-37. doi: 10.1016/j.ymeth.2010.05.004
40. Xu M, Ermolenkov VV, He W, Uversky VN, Fredriksen L, Lednev IK. Lysozyme fibrillation: Deep UV Raman spectroscopic characterization of protein structural transformation. Biopolymers. 2005; 79(1): 58-61. doi: 10.1002/bip.20330
41. Popova LA, Kodali R, Wetzel R, Lednev IK. Structural variations in the cross-β core of amyloid β fibrils revealed by deep UV resonance Raman spectroscopy. J Am Chem Soc. 2010; 132(18): 6324-6328. doi: 10.1021/ja909074j
42. Shashilov VA, Lednev IK. 2D correlation deep UV resonance raman spectroscopy of early events of lysozyme fibrillation: kinetic mechanism and potential interpretation pitfalls. J Am Chem Soc. 2008; 130(1): 309-317. doi: 10.1021/ja076225s
43. Xu M, Ermolenkov VV, Uversky VN, Lednev IK. Hen egg white lysozyme fibrillation: A deep-UV resonance Raman spectroscopic study. J Biophotonics. 2008; 1(3): 215-229. doi: 10.1002/jbio.200710013
44. Xu M, Shashilov V, Lednev IK. Probing the cross-β core structure of amyloid fibrils by hydrogen−deuterium exchange deep ultraviolet resonance Raman spectroscopy. Journal of the American Chemical Society. 2007; 129(36): 11002-11003. doi: 10.1021/ ja073798w
45. Manoharan R, Ghiamati E, Chadha S, Nelson W, Sperry J. Effect of cultural conditions on deep UV resonance Raman spectra of bacteria. Applied Spectroscopy. 1993; 47(12): 2145-2150. doi: 10.1366/0003702934066424
46. Chadha S, Manoharan R, Moënne-Loccoz P, Nelson W, Peticolas W, Sperry J. Comparison of the UV resonance Raman spectra of bacteria, bacterial cell walls, and ribosomes excited in the deep UV. Applied Spectroscopy. 1993; 47(1): 38-43. doi: 10.1366/0003702934048505
47. López-Díez EC, Goodacre R. Characterization of microorganisms using UV resonance Raman spectroscopy and chemometrics. Analytical Chemistry. 2004; 76(3): 585-591. doi: 10.1021/ ac035110d
48. Lakowicz JR. Principles of Fluorescence Spectroscopy 3rd ed. New York City, USA: Springer Science; 2011.
49. Yang S, Li B, Slipchenko MN, et al. Laser wavelength dependence of background fluorescence in Raman spectroscopic analysis of synovial fluid from symptomatic joints. J Raman Spectrosc. 2013; 44(8): 1089-1095. doi: 10.1002/jrs.4338
50. Min Y-K, Yamamoto T, Kohda E, Ito T, Hamaguchi H-o. 1064 nm near-infrared multichannel Raman spectroscopy of fresh human lung tissues. Journal of Raman Spectroscopy. 2005; 36(1): 73-76. doi: 10.1002/jrs.1280
51. Amigo JM, Babamoradi H, Elcoroaristizabal S. Hyperspectral image analysis. A tutorial. Anal Chim Acta. 2015; 896: 34-51. doi: 10.1016/j.aca.2015.09.030
52. Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro. 1978; 14(11): 911-915. doi: 10.1007/BF02616120
53. Brinkley B, Beall P, Wible L, Mace M, Turner DS, Cailleau R. Variations in cell form and cytoskeleton in human breast carcinoma cells in vitro. Cancer Res. 1980; 40(9): 3118-3129.
54. Winnard Jr PT, Zhang C, Vesuna F, et al. Organ-specific isogenic metastatic breast cancer cell lines exhibit distinct Raman spectral signatures and metabolomes. Oncotarget. 2017; 8(12): 20266-20287. doi: 10.18632/oncotarget.14865
55. Joo KM, Park IH, Shin JY, et al. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol Ther. 2009; 17(3): 570-575. doi: 10.1038/mt.2008.290
56. Lednev IK, Ermolenkov VV, He W, Xu M. Deep-UV Raman spectrometer tunable between 193 and 205 nm for structural characterization of proteins. Anal Bioanal Chem. 2005; 381(2): 431-437. doi: 10.1007/s00216-004-2991-5
57. Talari ACS, Movasaghi Z, Rehman S, Rehman IU. Raman spectroscopy of biological tissues. Applied Spectroscopy Reviews. 2015; 50(1): 46-111. doi: 10.1080/05704928.2014.923902
58. Stone N, Kendall C, Shepherd N, Crow P, Barr H. Nearinfrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. Journal of Raman Spectroscopy. 2002; 33(7): 564-573. doi: 10.1002/jrs.882
59. Bonnier F, Byrne H. Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems. Analyst. 2012; 137(2): 322-332. doi: 10.1039/c1an15821j
60. Ruiz-Chica A, Medina M, Sánchez-Jiménez F, Ramírez F. Characterization by Raman spectroscopy of conformational changes on guanine–cytosine and adenine–thymine oligonucleotides induced by aminooxy analogues of spermidine. Journal of Raman Spectroscopy. 2004; 35(2): 93-100. doi: 10.1002/jrs.1107
61. Chan JW, Taylor DS, Zwerdling T, Lane SM, Ihara K, Huser T. Micro-Raman spectroscopy detects individual neoplastic and normal hematopoietic cells. Biophys J. 2006; 90(2): 648-656. doi: 10.1529/biophysj.105.066761
62. Bhattacharjee T, Kumar P, Maru G, Ingle A, Krishna CM. Swiss bare mice: A suitable model for transcutaneous in vivo Raman spectroscopic studies of breast cancer. Lasers Med Sci. 2014; 29(1): 325-333. doi: 10.1007/s10103-013-1347-9
63. Dukor RK. Vibrational Spectroscopy in the Detection of Cancer. In: Handbook of Vibrational Spectroscopy. Hoboken, New Jersey, USA: John Wiley & Sons, Ltd; 2006.doi: 10.1002/0470027320.s8107
64. Gaudreau P-O, Stagg J, Soulières D, Saad F. The present and future of biomarkers in prostate cancer: Proteomics, genomics, and immunology Advancements. Biomarkers Cancer. 2016; 8: (Suppl 2): 15–33. doi: 10.4137/BIC.S31802
65. Filella X, Fernández-Galan E, Bonifacio RF, Foj L. Emerging biomarkers in the diagnosis of prostate cancer. Pharmgenomics Pers Med. 2018; 11: 83-94. doi: 10.2147/PGPM.S136026
66. Burns-Cox N, Avery NC, Gingell JC, Bailey AJ. Changes in collagen metabolism in prostate cancer: A host response that may alter progression. J Urol. 2001; 166(5): 1698-1701. doi: 10.1016/ S0022-5347(05)65656-X
67. Hall CL, Dai J, van Golen KL, Keller ET, Long MW. Type I collagen receptor (α2β1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Research. 2006; 66(17): 8648-8654. doi: 10.1158/0008-5472
68. Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (α2β1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008; 10(8): 797-803. doi: 10.1593/neo.08380
69. Crow P, Stone N, Kendall CA, et al. The use of Raman spectroscopy to identify and grade prostatic adenocarcinoma in vitro. Br J Cancer. 2003; 89: 106-108. doi: 10.1038/sj.bjc.6601059
70. Crow P, Barrass B, Kendall C, et al. The use of Raman spectroscopy to differentiate between different prostatic adenocarcinoma cell lines. Br J Cancer. 2005; 92(12): 2166-2170. doi: 10.1038/sj.bjc.6602638
71. Prensner JR, Rubin MA, Wei JT, Chinnaiyan AM. Beyond PSA: The next generation of prostate cancer biomarkers. Sci Transl Med. 2012; 4(127): 127rv123. doi: 10.1126/scitranslmed.3003180
72. Shetty G, Kendall C, Shepherd N, Stone N, Barr H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br J Cancer. 2006; 94(10): 1460-1464. doi: 10.1038/sj.bjc.6603102
73. Stone N, Kendall C, Smith J, Crow P, Barr H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004; 126: 141-157. doi: 10.1038/sj.bjc.6603102
74. Holdhoff M, Yovino SG, Boadu O, Grossman SA. Bloodbased biomarkers for malignant gliomas. J Neurooncol. 2013; 113(3): 345-352. doi: 10.1007/s11060-013-1144-0
75. Khalil AA. Biomarker discovery: A proteomic approach for brain cancer profiling. Cancer Sci. 2007; 98(2): 201-213. doi: 10.1111/j.1349-7006.2007.00374.x
76. Schwartz SA, Weil RJ, Johnson MD, Toms SA, Caprioli RM. Protein profiling in brain tumors using mass spectrometry: Feasibility of a new technique for the analysis of protein expression. Clin Cancer Res. 2004; 10(3): 981-987. doi: 10.1158/1078-0432. CCR-0927-3
77. Kendall C, Day J, Hutchings J, et al. Evaluation of Raman probe for oesophageal cancer diagnostics. Analyst. 2010; 135(12): 3038-3041. doi: 10.1039/c0an00536c
78. Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proceedings of the National Academy of Sciences. 2008; 105(5): 1410-1415. doi: 10.1073/pnas.0707654105
79. Huang Z, McWilliams A, Lui H, McLean DI, Lam S, Zeng H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int J Cancer. 2003; 107(6): 1047-1052. doi: 10.1002/ ijc.11500
80. Liu T, Chen C, Shi X, Liu C. Evaluation of Raman spectra of human brain tumor tissue using the learning vector quantization neural network. Laser Physics. 2016; 26(5): 055606.doi: 10.1088/1054-660X/26/5/055606
81. Kalkanis SN, Kast RE, Rosenblum ML, et al. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J Neurooncol. 2014; 116(3): 477-485. doi: 10.1007/s11060-013-1326-9
82. Dabholkar MD, Berger MS, Vionnet JA, et al. Malignant and nonmalignant brain tissues differ in their messenger RNA expression patterns for ERCC1 and ERCC2. Cancer Res. 1995; 55(6): 1261-1266.
83. Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015; 121(1): 101-108. doi: 10.1007/s11060-014-1613-0
84. Mahadevan-Jansen A, Richards-Kortum R. Raman spectroscopy for cancer detection: A review. Paper Presented at: Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 30 Oct.-2 Nov, 1997; Chicago, IL, USA: doi: 10.1109/IEMBS.1997.756895
85. Bocklitz TW, Guo S, Ryabchykov O, Vogler N, Popp Jr. Raman based molecular imaging and analytics: A magic bullet for biomedical applications? Anal Chem. 2016; 88(1): 133-151. doi: 10.1021/acs.analchem.5b04665
86. Lednev IK. Biological Applications of Ultraviolet Raman Spectroscopy. In: Uversky VN, Permyakov EA, eds. Methods in Protein Structure and Stability Analysis: Vibrational Spectroscopy. Hauppauge, NY, USA: Nova Science Publishers, Inc.; 2007: 1-26
87. Beaven G, Holiday E. Ultraviolet absorption spectra of proteins and amino acids. Advances in Protein Chemistry. 1952; 7: 319- 386. doi: 10.1016/S0065-3233(08)60022-4
88. Wen ZQ, Thomas Jr GJ. UV resonance Raman spectroscopy of DNA and protein constituents of viruses: Assignments and cross sections for excitations at 257, 244, 238, and 229 nm. Biopolymers. 1998; 45(3): 247-256. doi: 10.1002/(SICI)1097- 0282(199803)45:3<_x0032_47:_x003a_AID-BIP7>3.0.CO;2-R
89. Fodor SP, Copeland RA, Grygon CA, Spiro TG. Deep-ultraviolet Raman excitation profiles and vibronic scattering mechanisms of phenylalanine, tyrosine, and tryptophan. Journal of the American Chemical Society. 1989; 111(15): 5509-5518. doi: 10.1021/ ja00197a001
90. Ziegler L, Hudson B, Strommen D, Peticolas W. Resonance Raman spectra of mononucleotides obtained with 266 and 213 nm ultraviolet radiation. Biopolymers. 1984; 23(10): 2067-2081. doi: 10.1002/bip.360231017
91. Fodor SP, Rava RP, Hays TR, Spiro TG. Ultraviolet resonance Raman spectroscopy of the nucleotides with 266-, 240-, 218-, and 200-nm pulsed laser excitation. Journal of the American Chemical Society. 1985; 107(6): 1520-1529. doi: 10.1021/ja00292a012
92. Voet D, Gratzer W, Cox R, Doty P. Absorption spectra of nucleotides, polynucleotides, and nucleic acids in the far ultraviolet. Biopolymers. 1963; 1(3): 193-208. doi: 10.1002/bip.360010302
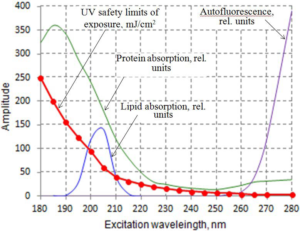
93. International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation). Health Phys. 2004; 87(2): 171-186. doi: 10.1097/00004032-200408000-00006
94. Monici M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu Rev. 2005; 11: 227-256. doi: 10.1016/S1387-2656(05)11007-2
95. Jamme F, Kascakova S, Villette S, et al. Deep UV autofluorescence microscopy for cell biology and tissue histology. Biol Cell. 2013; 105(7): 277-288. doi: 10.1111/boc.201200075
96. Cheng W-T, Liu M-T, Liu H-N, Lin S-Y. Micro-Raman spectroscopy used to identify and grade human skin pilomatrixoma. Microsc Res Tech. 2005; 68(2): 75-79. doi: 10.1002/jemt.20229
97. Kirschenbaum DM. Molar absorptivity and A 1% 1cm values for proteins at selected wavelengths of the ultraviolet and visible regions. XIII. Anal Biochem. 1977; 81(1): 220-246.doi: 10.1016/00032697(77)90137-3
98. Angioni E, Lercker G, Frega NG, et al. UV spectral properties of lipids as a tool for their identification. European Journal of Lipid Science and Technology. 2002; 104(1): 59-64. doi: 10.1002/1438-9312(200201)104:1<_x0035_9:_x003a_AID-EJLT59>3.0.CO;2-I
99. Bykov VJ, Hemminki K. Assay of different photoproducts after UVA, B and C irradiation of DNA and human skin explants. Carcinogenesis. 1996; 17(9): 1949-1955.doi: 10.1093/carcin/17.9.1949
100. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014; 21(2): 260-292. doi: 10.1089/ars.2013.5489
101. Gršković B, Zrnec D, Popović M, Petek MJ, Primorac D, Mršić G. Effect of ultraviolet C radiation on biological samples. Croat Med J. 2013; 54(3): 263-271. doi: 10.3325/cmj.2013.54.263